Yuliang Zhao, Bing Wang, Weiyue Feng, Chunli Bai
NANOTOXICOLOGY- TOXICOLOGICAL ACTIVITIES OF NANOMATERIALS
AND BIOLOGICAL
Yuliang Zhao, CAS Key Lab for Biomedical Effects of Nanomaterials and Nanosafety, Institute of High Energy Physics, The Chinese Academy of Sciences, Beijing 100049, & National Center for Nanoscience and Technology of China, Beijing 100190, China Bing Wang, CAS Key Lab for Biomedical Effects of Nanomaterials and Nanosafety, Institute of High Energy Physics, The Chinese Academy of Sciences, Beijing 100049
Chunli Bai National Center for Nanoscience and Technology of China, Beijing 100190, China The Chinese Academy of Sciences, Beijing 100864, China Keywords: Nanotoxicology, Nanosafety, Nanomaterials, Nanoparticles, Contents
1. Introduction 2. Target organ toxicity of nanoparticles 2.1. Respiratory System 2.1.1. Deposition of Nanoparticles in the Respiratory Tract 2.1.2. Clearance of Nanoparticles in the Respiratory Tract 2.1.3. Nanotoxic Response of Respiratory System 2.2. Gastrointestinal System 2.3. Cardiovascular System 2.4. Central Nervous System 2.5. Skin 3. Absorption, distribution, metabolism and excretion of nanoparticles (ADME) 3.1. ADME of Nanoparticle Following Inhalation Exposure 3.1.1. Absorption and Retention of Nanoparticles Following Respiratory Tract Exposure 3.1.2. Translocation and Distribution of Nanoparticles Following Respiratory Tract Exposure 3.1.3. Metabolism and Excretion of Nanoparticles in the Lung 3.2. ADME of Nanoparticle via Gastrointestinal Tract 3.3. ADME of Nanoparticles via Skin 4. Cytotoxicity of nanoparticles 4.1. The Interaction of Metallic and Metal Oxide Nanoparticles with Cells 4.2. The Cytotoxicity of the Metallic Nanoparticles and the Possible Mechanism 5. Molecular toxicology of nanoparticles 5.1. The Interaction of Nanoparticles with DNA 5.2. The Interaction of Nanoparticles with Proteins
Glossary Bibliography Biographical Sketches Summary All aspects of our life will benefit from the revolution in nanotechnology. This revolution will necessitate large scale production of nanosize particles/structures, new formulations and novel surface properties to meet demands of novel functions. Meanwhile, the rapidly development of nanotechnology is likely to become new sources of human or environmental hazards through inhalation, ingestion, skin uptake, or injection of engineered nanomaterials in workplace or the use of consumer products. So far, research results suggest that nanoparticles may cause adverse effects on human health at their portal of entry, which show difference from the bulk materials of the same chemical composition. Taking the lung as an example, some nanoparticles can escape the normal defenses and translocate from their portal of entry to induce diverse impacts in other organs, and in some cases, the nanoparticles stay in the organ for a long time and be not easily excreted from the body.[F1] On interaction at a cellular level, some nanoparticles easily enter into the cells, etc. In order to gain a sustainable development, new technology always needs a good balance between the benefit and risk. Nanotoxicology is intended to address the toxicological activities of nanoparticles and their products to determine whether and to what extent they may pose a threat to the environment and to human health, and defined as the study of the nature and mechanism of toxic effects of nanoscale materials/particles on living organisms and other biological systems. It also deals with the quantitative assessment of the severity and frequency of nanotoxic effects in relation to the exposure of the organisms. The knowledge from nanotoxicological study will be the base for designing safe nanomaterials and nanoproducts, and also direct uses in the nanomedical sciences. This chapter consists of five sections with a brief introduction, the target organ toxicity of nanoparticles in different biological systems, the ADME (absorption, distribution, metabolism and excretion) of nanoparticles in vivo following different exposure routs, the cytotoxicity and molecular nanotoxicology of nanoparticles. 1. Introduction
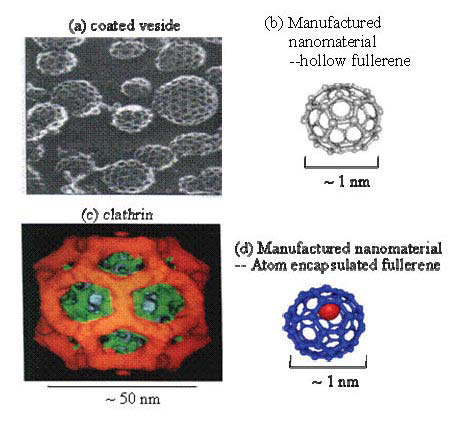
With the fast development of nanotechnology, industries are currently involved in nanotechnology-related activities, among which the manufactured nanoscale materials or engineering nanoparticles are using in a wide range of products. It is known that nanostructured materials or nanoscale particles possess many novel properties such as self-assembly, size effects, large surface area, ultrahigh reactivity and quantum effects because of their very small size and unique structures. According to data collected by the National Nanotechnology Initiative (NNI), the quantity of manufactured nanoscale material is growing significantly every year. Business Communications Company has projected a $10 billion global demand for nanoscale materials, tools, and devices in 2010 (see website: http://www.nano.gov/). This large increase in demand and production could lead to enormous exposures of humans and other organisms to nanomaterials/nanoparticles. What happens with these very small-size materials when they entered our body, in particular what can be expected with the increased surface reactivity which may lead to completely different biological effects in vivo as compared to the bulk material of the identical chemical composition and the same quantity. Of cause they can either be positive and desirable, or negative and undesirable, or a mix of both, these mostly depend on how to utilize them. Nanotoxicology is defined as the study of the nature and mechanism of toxic effects of nanoscale materials/particles on living organisms and other biological systems. It also deals with the quantitative assessment of the severity and frequency of nanotoxic effects in relation to the exposure of the organisms. In fact, scientists have studied the healthy effects of exposure to airborne nanoparticles for years and have shown some unexpectedly adverse health effects of nanosized particles in vivo. For instance, the epidemiological investigations have found the associations of incidence of a disease and mortality with the concentrations and the sizes of the airborne particles in the environment[F2]. The increase of mortality might result from the abundant increase of nanoparticles. Recently, the biological/toxicological effects of manufactured nanomaterials and nanoparticles have attracted much attention and been seriously discussed (Service, 2003; Kelly, 2004; Brumfiel, 2003; Zhao et al., 2008). From the fact that the sizes of the nanoparticle and the biological molecule are comparable (Figure1), one easily bethinks of such a consequence that the nanoparticle may easily invade the natural defense system of human body or other species and easily enter the cells to affect cellular functions. The existing knowledge reminds that when molecules are small enough, it is possible for them to slip past the guardians in our respiratory systems, skip through our skin into unsuspecting cells, and (sometimes) cross through the blood-brain barrier (BBB). More essentially, the life process is mainly held out by a series of biochemical reactions in vivo. Manufactured nanoparticles possess ultra huge surface area, ultrahigh reactivity, highly efficient catalysis, and nanostructures, etc., do they interfere the normal biochemical reactions in vivo when entering the human body? Are these interferences beneficial or harmful to the life process? How to avoid the harmful effects, and how to utilize the beneficial effects? For instance, one sees the similarity in geometric structures of manufactured nanocage (fullerene) and the biological protein (clathrin) in Figure 1, they all consist of the pentagon and hexagon rings. In fact, many knowledge gaps need to be filled in. For instance, the large alteration in physicochemical properties of nanomaterials as compared to the bulk material of the same chemical form, and the large alteration in physicochemical characteristics between different sizes of the same nanomaterials will undoubtedly lead to different biological effects in vivo. So the existing database of safety evaluation for the bulk materials, including the effects on health and the environment is probably no longer valid when extrapolating and applying them for the safety assessment of nanomaterials.
[F3]In any application or even at the initial stages of the research and development, these nanoparticles easily enter the environment via various routes, and ultimately enter human body through direct routes such as dermal and oral exposures, nanodrugs, or through indirect routes such as the food chain, etc. For nanoparticles, except the very strong size-effects, other unique properties such as the tremendous surface, anomalous interface, complicated reactivity, quantum effects, etc. can also lead to changes in physicochemical properties which naturally alter the biological activities in vivo[F4]. These have been demonstrated by the reported data and will be summarized and discussed in the following sections of this chapter (Warheit et al., 2004; Lam, 2004; Chen, 2007; Feng et al., 2009; Fischer et al., 2007; Wang et al., 2005; Zhao et al., 2007; Nel et al., 2009). Thus, in this chapter, the toxicological effects of nanomaterials will be clarified in detail at whole-animal, cellular and molecular levels based on the experimental findings from both the in vivo to in vitro models. 2. Target Organ Toxicity of Nanoparticles As different tissues and organs have different compositions, structures and functions, toxic responses are mostly different once nanoparticles (NPs) enter different organs. Human skin, intestinal tract and respiratory tract are always in direct contact with the environmental nanoparticles. For instance, skin, as a structural barrier between the environment and the body, plays an important role to protect against break-in of exogenic particles. Respiratory tract,
generally divided into three segments, upper-----respiratory tract, respiratory airways and lung, most of which exists merely as a piping system for air to travel in the lungs. Gastrointestinal tract, also known as the digestive tract, can uptake, transport, digest and adsorb various substances such as nutrients, water and vitamins from food. On the other hand, the gastrointestinal tract is also designed as a barrier to restrain the entry of pathogens, toxins and undigested macromolecules. As such, these potential exposure routes are likely to be the first portal of entry for nanoparticles invading into the human body. In addition, other physiological systems such as cardiovascular system and central nervous system may have chances to interact with exogenous nanoparticles circulated or transported from the above exposure routes. So, they may also be a specific target for any type of nanoparticle into the human body. 2.1. Respiratory System We may say that we inaugurate our life with an inspiration of air. However, the air we breathe is not so pure as we would desire it to be, usually containing other pollutants such as by-products of the combustion processes related to fires, heating, industrial and automotive processes. So, the toxicity research on NPs in vivo has been carried out on respiratory system of mammalian. 2.1.1. Deposition of Nanoparticles in the Respiratory Tract
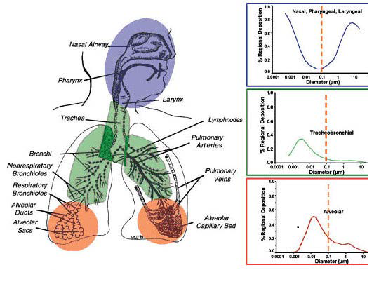
Respiratory system can be further divided into different target zones such as nasopharyngeal, tracheobronchial and alveolar regions. Specific defense mechanisms may protect the mammalian organism from harmful materials at the portal of entry, however, these defenses may not always be as effective for NPs. When the NPs are inhaled, their deposition, clearance, and translocation within the respiratory tract will be different from the larger particles.
According to the diffusion motion, exposure to airborne NPs via the inhalation route will deposit throughout the entire respiratory tract, starting from nasopharyngeal and tracheobronchial, down to the alveolar regions. Depending on the particle size, the deposition fractions of inhaled NPs in these regions show significant difference. For instance, 90% of inhaled 1 nm particles are deposited in the nasopharyngeal compartment, only ~10% in the tracheobronchial region, and essentially none in the alveolar region. On the other hand, 5 nm particles show about equal deposition of ~30% of the inhaled particles in all three regions; 20 nm particles have the highest deposition efficiency in the alveolar region (~50%), whereas in tracheobronchial and nasopharyngeal regions this particle size deposits with ~15% efficiency (Figure2) (ICRP, 1994). These different deposition efficiencies should have consequences for potential effects induced by inhaled NPs as well as for their translocation beyond the respiratory tract. 2.1.2. Clearance of Nanoparticles in the Respiratory Tract Pulmonary retention and clearance of microsized particles have been studied for many years. While, the recent studies on occupational and environmental exposure of nanoparticles (NPs) generated a considerable amount of new knowledge regarding the clearance of NPs in the respiratory tract. The clearance of deposited particles in the respiratory tract is mainly by physical translocation to other sites or chemical clearance. Chemical dissolution in the upper or lower respiratory tract occurs for biosoluble NPs in the intra-cellular or extra-cellular fluids. The solutes and soluble components can then undergo absorption and diffusion in other subcellular structures or binding to proteins in cells and may be eventually cleared into blood and lymphatic circulation. This clearance mechanism can happen at any location within the three regions of the respiratory tract, depending on the pH of local extracellular and intracellular compartments. The clearance for insoluble or poorly soluble NPs in the respiratory tract is basically via physical translocation. The efficiency of this clearance depends highly on the site of deposition and particle size. The NPs trapped within the mucocillary escalator from the upper airways (nasopharyngeal and tracheobronchial) is expelled by pushing the mucus toward the mouth, hence, it is a relatively rapid process[F5] (Kreyling and Scheuch, 2000). For NPs within the alveolar regions, the most prevalent clearance is mediated by macrophage phagocytosis, which highly depends on the efficiency of alveolar macrophages to "sense" deposited particles, move to the site of their deposition, phagocytize them, and then move towards the mucociliary escalator (Kreyling and Scheuch, 2000). 2.1.3. Nanotoxic Response of Respiratory System
The epidemiological studies have found a correlation between exposure to respirable airborne particulate matter (PM) and increased mortality and adverse respiratory health effects, including the development of emphysema, chronic bronchitis, and asthma (Samet, 2000; Hoet, 2004). As the most toxic component of airborne particulate matter, nanoparticles have uncontrolled access to the cells of the airway and even intracellular components because of their size. Hence, deposition of NPs in the alveolar spaces of the lung plays a central role to pulmonary toxicity. When inhaled, NPs deposit dispersedly upon the alveolar surface, which likely leads to a scattered chemoattractant signal, resulting in lower recognition and alveolar macrophage responses (Kreyling et al., 2006). The biopersistence of inhaled particles is an important characteristic dictating the level of inflammation and tissue injury. The representative studies of pulmonary inflammation and fibrosis induced by nanoparticles in experimental animal was first reported by Lam and Warheit who demonstrated that intratracheal instillation of single-walled carbon nanotube (SWNTs) and multi-walled carbon nanotube (MWNTs) under a certain dose induced a pulmonary granuloma formation and some interstitial inflammation (Figure3) (Lam, 2004; Warheit, 2004). Further study indicates both SWNTs and MWNTs could induce the alteration of cell structures (Jia et al., 2005). For instance, when exposed to 5 g/mL SWNTs, the macrophage cell exhibited condensed folds, while the nucleus degenerated and the nuclear matrix reduced when treated with MWNTs (Figure4). At a higher dose level of 20 g/mL, cells exposed to SWNTs became swelled and vacuolar, and presented phagosomes explicitly; while in those exposed to MWNTs, the chromatin was concentrated, selenodont border and vacuole in cytosol were presented. All the above alterations show signs of cell apoptosis. It is important to note that the cell apoptosis induced by SWNTs and MWNTs under a certain dose is much different from the cell necrosis, which was accompanied with inflammation. Recent studies on intratracheal instillation of nanoparticles in rats showed intratracheally instilled ferric oxide nanoparticles (20 nm) induced some clinical pathological changes such as follicular hyperplasia, protein effusion, pulmonary capillary vessel hyperaemia and alveolar lipoproteinosis in lung [F6] Figure5 . The sustain burden of particles in alveolar macrophages and epithelium cells has caused lung emphysema and pro-sign of lung fibrosis (Zhu et al., 2008). Most recently, an age-related difference in the pulmonary response to the inhaled SiO2 nanoparticles has been found, i.e. the same respiratory exposure caused much severer pulmonary inflammation in old rats than in young or adult rats[F7].
Figure 3. Lung tissue from mice instilled with 0.5 mg of a test material per mouse and killed 7 d after the single treatment. (a) Serum control; (b) carbon black (CarboLex nanotubes); (c) silica quartz; (d) CNT; (e) RNT (raw nanotube); (f) PNT (purified nanotube).---Figure 4. Ultrastructural changes of phagocytes induced by SWNTs and MWNTs (with diameter of 10-20 nm) particles at different doses (Jia et al., 2005). (a) Control; (b) control; (c) 5 g/mL SWNTs; (d) 20 g/mL SWNTs; (e) 5 g/mL MWNTs; (f) 20 g/mL MWNTs.
Figure 5. Histopathology of lung at days 7 and 30 after intratracheal instillation of particles or saline (hematoxylineosin stain, magnification = 50). Follicular hyperplasia of the lymph node was formed at trachea forks (dark arrow). (A) Control group; (B) days 7 after instillation of 0.8 mg/kg bw 22 nm-Fe2O3; (C) days 30 after instillation of 0.8 mg/kg bw22 nm-Fe2O3; (D) days 7 after instillation of 0.8 mg/kg bw280 nm-Fe2O3; (E) days 30 after instillation of 0.8 mg/kg bw 280 nm-Fe2O3; (F) days 7 after instillation of 20 mg/kg bw22 nm-Fe2O3; (G) days 30 after instillation of 20 mg/kg bw22 nmFe2O3; (H) days 7 after instillation of 20 mg/kg bw 280 nm-Fe2O3; (I) days 30 after instillation of 20 mg/kg bw 280 nm-Fe2O3. (Zhu et al., 2008)
2.2. Gastrointestinal System The gastrointestinal tract is one of the largest immunological organs of the body, containing more lymphocytes and plasma cells than the spleen, bone marrow and lymph nodes. It is considered that the exogenous sources of ingestion exposure primarily results from hand-to-mouth contact in the workplace. Alternatively, NPs can be ingested directly via food, water, drinking, drugs or drug delivery systems. In addition, NPs cleared from the respiratory tract via the mucociliary escalator can subsequently be ingested into the gastrointestinal (GI) tract. Thus, gastrointestinal tract is considered as an important target for NPs exposure.
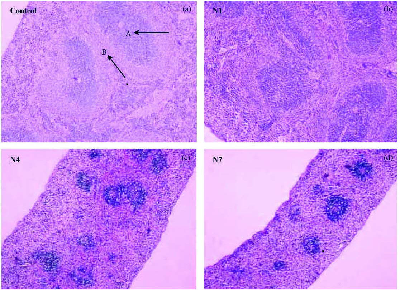
Figure 6. The microscopic pictures (×100) [F9]show the pathological changes in kidney tissues of experimental mice of the control (a); 500 mg/kg nano-copper exposed group (b); 1851 mg/kg nano-copper exposed group (c) 5000 mg/kg nano-copper exposed group (d). A: renal glomerulus and B: Bowman's capsule. So far, our research group has investigated the acute oral toxicity of several types of nanoparticles with gastrointestinal tract exposure. For instance, we compared the oral toxicity of copper nanoparticles (23.5 nm) and micro particles (17 m) in mice (Chen et al. 2006). In 17 m particles treated mice only few mice exhibited symptoms of poising, however, all by nano-copper treated mice appeared obviously symptoms of alimentary canal function disorder, such as loss of appetite, diarrhea and vomiting, etc. Further pathological examination revealed grave injuries on kidney, liver and spleen in mice exposed to nano-copper particles, but not found in mice exposed to micro-copper-particles on mass basis[F10] (Figure6-Figure8). When mice were orally administrated with 20 nm and 120 nm ZnO NPs at different doses, we found that the liver, spleen, heart, pancreas and bone became the target organs whose damages show different doseresponse relationship (Wang et al., 2008). The 120 nm ZnO treated mice had a positive dose-effect pathological damage in stomach (inflammation in gastric lamina propria, submucosa or serosa layer), liver (fatty degeneration of hepatocytes around central vein or portal area), heart (fatty degeneration of cardiovascular cells) and spleen (largement of splenic corpuscle), whereas, 20nm ZnO displayed a negative dose-effect damage in the above mentioned organs.--Figure 7. The microscopic pictures (×200) [F11]show the pathological changes in liver tissues of experimental mice of the control (a); 500 mg/kg nano-copper exposed group (b); 1851 mg/kg nano-copper exposed group (c) and 5000 mg/kg nano-copper exposed group (d). A: steatosis.--Figure 8. The microscopic pictures (×40) show the pathological changes in spleen tissues of experimental mice of the control[F12] (a); 500 mg/kg nano-copper exposed group (b); 1851 mg/kg nano-copper exposed group (c) and 5000 mg/kg nano-copper exposed group (d). A: splenic unit and B: lymphocytes. 2.3. Cardiovascular System
The cardiovascular system is composed of the heart, blood vessels, vasculature, the cells and plasma that make up the blood. The principal function of the heart is to continuously pump blood around the cardiovascular system. It receives both sympathetic and parasympathetic nerve fibres which alter the rate of the beat, but they do not initiate the contraction. The blood vessels of the body represent a closed delivery system, which functions to transport blood around the body, circulating substances such as oxygen, carbon dioxide, nutrients, hormones and waste products. The epidemiologic investigations have shown a direct credible relationship between ambient air particulate pollution and a consistent association with increased health effects specifically leading to cardiovascular diseases. The concentration response relationship between PM2.5 and daily deaths was reported to cause 100,000 deaths annually in the United States (Schwartz et al., 2002). In a recent comprehensive review of epidemiologic studies it was shown clearly the pathophysiological changes of cardiovascular diseases had close association with exposure to ultrafine particles (UFPs) in air. In the case of manufactured nanoparticles, a single intrapharyngeal instillation of single-
From above findings about cardiovascular response to nanoparticles exposure, one may raise a new and significant question: whether the health effects of air particulate is dominated by the nanosized fraction in air. Moreover, besides the factor of size, other physicochemical parameters of nanoparticles, such as shape, crystal structure, solubility, surface area, surface charge, surface coating may also play key roles on the cardiovascular events,
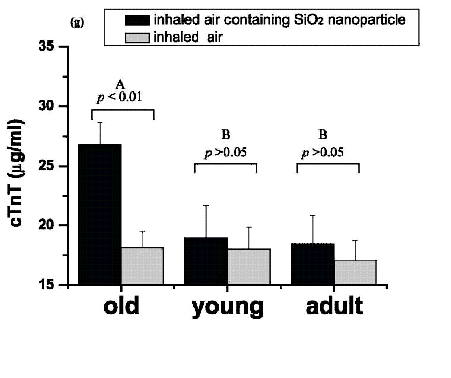
Figure 9. With identical inhalation of nanoparticles, myocardial ischemic damage was seen only in older rats. wall carbon nanotubes (SWCNTs) can induce activation of heme oxygenase-1 (HO-1), a marker of oxidative insults, in lung, aorta, and heart tissue in HO-1 reporter transgenic mice (Li et al. 2007). Furthermore, the C57BL/6 mice exposed to SWCNT (dose: 10 and 40 g/mouse) developed some pathophysiological changes related to cardiovascular diseases such as mitochondria DNA damage, elevation of mitochondrial glutathione and protein carbonyl levels (Li et al., 2007). In order to study the age-related difference in cardiovascular responses to SiO2 nanoparticles inhalation exposure, we designed a novel nanoparticles exposure system, a sealed Plexiglas exposure chamber specifically mimicking natural (physiologic) inhalation, and investigated the toxicity sensitivity of nanoparticles in different ages (young, adult, old) of rats (Chen et al., 2008). We measured and analyzed the changes in serum biomarkers, hemorheologic, heart injury, and pathology in rats of different ages, and found that the SiO2 nanoparticles inhalation under identical conditions caused severe myocardial ischemia, significant elevation of blood viscosity and fibrinogen concentration in old rats, yet less change in young and adult rats. The results indicate that old individuals are more sensitive to nanoparticle exposure than the young and adult rats (Chen et al., 2008) (Figure9). -Figure10. Changes in whole blood viscosity (b) in different-aged rats who inhaled air containing manufactured SiO2 nanoparticles. b (mean ( SD) is plotted against shear rate at intervals from 1 S1- to 200 S1- and hematocrit at 41%. b was significantly elevated in the exposed group of old rats [F13] (a), but no statistical differences were observed between the exposed and control groups in each of the young (b) and adult (c) groups. Two asterisks (**) represents p < 0.01 in the one-way ANOVA t-test. Panels d and e show the changes in fibrinogen and plasma viscosity (p) as a function of age. p, A, and B are defined in Figure 2. A and B are the Duncan class of Duncan's multiple-range test. (Chen et al., 2008) 2.4.
Central Nervous System The central nervous system (CNS), consisting of the brain and spinal cord, is responsible for receiving and interpreting signals from the peripheral nervous system and also sends out signals to it, either consciously or unconsciously. Although respiratory system is considered to be the main portal of entry for inhaled nanoparticles, extrapulmonary translocation after respiratory tract deposition is likely to happen via accidental or occupational acute exposure (Nemmar et al., 2002). It is also possible that--inhaled UFPs, by virtue of their extremely small size may deposit in the olfactory mucosa and then translocate in the central nervous system, which in turn might cause neurotoxicity. Recent studies support the concept that the CNS may be an important target organ for nanoparticle inhalation or intranasal instillation exposure (Oberdörster et al., 2004; Elder et al., 2006). The inhaled ultrafine carbon (35 nm) and manganese oxide nanoparticles (30 nm) can translocate in the brain via the olfactory neuronal pathway [F14] (Oberdörster et al., 2004; Elder et al., 2006). In particular, some investigations have indicated that inhaled or intranasally instilled ultrafine particle may trigger a proinflammatory response in nervous tissue. For instance, intranasally instilled ultrafine carbon black (14 nm) can induce inflammatory changes (interleukin-1, tumor necrosis factor- and chemokines mRNA) in the brain olfactory bulb [F15] (Tin-Tin-Win-Shwe et al., 2006). Further, the size dependent potential damage of nanoparticles on CNS was also demonstrated by the study of intranasal instillation of carbon black (CB) nanoparticles in mice. The proinflammatory responses were observed in brain olfactory bulb of the 14nm CB treated mice but not in the 95nm CB treated ones. The intranasal instillation of ufCB may influence the brain immune function depending on their size. (Tin-Tin-Win-Shwe et al., 2006). In our recent study, we found a time-dependent translocation pattern and potential damages of TiO2 nanoparticles on CNS through intranasal instillation. We collected the brain tissues and measured the accumulation and distribution of TiO2 (Figure11). The tissues were analyzed with histopathology, oxidative stress, and inflammatory markers at post-instillation time points of 2, 10, 20 and 30 days[F16] . Results indicated that the instilled TiO2 directly entered the brain through olfactory bulb in the whole exposure period, especially deposited in the hippocampus region and induced pathological changes in the olfactory bulb and hippocampus regions[F17] (Wang et al., 2008).--Figure 11. SRXRF mapping of Ti-element distribution in the brain sections at 30 days after intranasal instillation of the different-sized TiO2 particles. In the control mice, the Ti contents are lower than the detection limit of SRXRF and the mapping is not available.-- 2.5. Skin The human skin, the largest organ in the body, is composed of three layers - epidermis, dermis and subcutaneous, protecting against the environment with a surface area of nearly 18,000 cm2. The outer portion of the epidermis, called stratum corneum is a 10 m thick keratinized layer of dead cells and is difficult to pass through by ionic compounds or water soluble molecules. The surface of epidermis is highly microstructured, having a scaly appearance as well as pores for sweat, sebaceous glands, and hair follicle sites. Skin is considered to be the barrier between the wellregulated ``milieu interieur'' and the outside environment. It also has a relative large surface area for exposure, serving as one of the principal portals of entry by which nanomaterials can enter the body. Currently, there is a lack of information on whether nanoparticles can be absorbed across the skin's stratum corneum barrier or whether systemically administered particles can accumulate in dermal tissue. The tendency for nanomaterials to traverse the skin is a primary determinant of its dermatotoxic potential. That is, the nanomaterials or nanoparticles must penetrate the uppermost stratum corneum layer in order to gain entrance to the viable epidermis and exert toxicity in the lower cell layers. Up to now, nanoparticle dermal penetration is still under controversial. A current area under discussion is whether or not TiO2 NPs in commercially available sunscreens can penetrate the skin to enter the body. Several studies in murine, porcine, or human skin have confirmed that TiO2 NPs remained on the skin surface or the outer layers of the skin and had not penetrated into or through the living skin (Lademann et al., 1999; Pflücker et al., 2001; Schulz et al., 2002). However, there is some evidence which suggests the NPs may penetrate into the epidermis or dermis. Bennat and MüllerGoymann (2000) applied TiO2 NPs to human skin either as an aqueous suspension or oil-in-water emulsion and evaluated skin penetration using the tape stripping method (Bennat and Müeller-Goymann, 2000). They observed that TiO2 NPs apparently penetrated deeper into human skin when applied as an oil-in-water emulsion, and that penetration was greater when applied to hairy skin, which suggest that TiO2 NPs penetrate surface through hair follicles or pores. Nanoparticles caused dermatoxicity was reported by both in vivo or in vitro experiments. In an animal model study, both 0.5- and 1.0-m beryllium particles could penetrate the stratum corneum and develop hapten-specific, cell-mediated immune responses [F18] (Tinkle et al. 2003). In an in vitro study, Shevedova et al. (2003a) reported that SWCNT caused a significant dose-response reduction of cell viability and oxidative stress biomarkers (e.g., antioxidant reserve), and a significant increase in lipid peroxides in human epidermal keratinocytes (0, 0.06, 0.12 and 0.24 mg/mL of SWCNT for 18 hours), suggesting an increase of cutaneous toxicity. In the recent years, a majority of nanotoxicity research is focusing on in vitro systems. However, the data from in vitro studies easily mislead the safety assessment efforts[F19] . The data will require verification from in vivo animal experiments. On the other hand, in vivo systems are extremely complicated and the interactions of the nanostructures with biological components, such as proteins and cells, could lead to unique biodistribution, clearance, immune response, and metabolism.[F20] Thus, an understanding of the--relationship between the physical and chemical properties of the nanostructure and their in vivo behavior would provide a basis for assessing nanotoxicity and more importantly may lead to predictive models for nanotoxicity assessment.
-
TO ACCESS ALL THE 53 PAGES OF THIS CHAPTER, Visit: http://www.eolss.net/Eolss-sampleAllChapter.aspx
Bibliography
Ahamed M., Karns M., Goodson M., Rowe J., Hussain S.M., Schlager J.J. (2008). DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol Appl Pharmacol 233, 404-410. [This paper reported the relationship between different prosperities of nanoparticles and cell damaging.] AO L.M., Gao F., Pan B.F., Cui D.X., Gu H.C. (2006). Interaction between gold nanoparticles and bovine serum albumin or sheep antirabbit immunoglobulin G. Chin J Chem 24, 253-256. [This paper studied Interaction between gold nanoparticles and proteins under different conditions.] Becker C., Hodenius M., Blendinger G., Sechi A., Hieronymus T., Muller-Schulte D., Schmitz-Rode T., Zenke M. (2007). Uptake of magnetic nanoparticles into cells for cell tracking. J Magn Magn Mater 311, 234-237. [This paper investigated cellular localization of nanoparticles using the fluorescent tag.] Bennat C., Mueller-Goymann C.C. (2000). Skin penetration and stabilization of formulations containing microfine titanium dioxide as physical UV filter. Int J Cosmet Sci 22, 271-283. [This article showed encapsulation of the micropigment into liposomes does not result in a better stability but it causes a higher penetration depth of the particles into the skin.] Bennat C., Mueller-Goymann C.C. (2000). Skin penetration and stabilization of formulations containing microfine titanium dioxide as physical UV filter. Int J Cosmet Sci 22, 271-283. [The article investigated the in vivo and in vitro penetration behavior of the physical UV filter into human skin] Berry J.P., Arnoux B., Stanislas G., Galle P., Chretien J. (1977).A microanalytic study of particles transport across the alveoli: role of blood platelets. Biomedicine 27, 354-357 [The article investigated the role of blood platelets in the particles' transport across the alveoli] Bodian D.H.H.A. (1941). The rate of progression of poliomyelitis virus in nerves. Bull Johns Hopkins Hosp 69, 79-85. [This article investigated the rate of progression of poliomyelitis virus in nerves via olfactory neuronal route] Borm P.J., Schins R.P., Albrecht C. (2004). Inhaled particles and lung cancer, part B: paradigms and risk assessment. Int J Cancer 110, 3-14. [This review discusses the current paradigms in rodent particle carcinogenicity, i.e., (i) role of particle overload and of persistent inflammation and (ii) fibrosis as an intermediate step in particle-induced lung cancer with regard to human risk assessment.] Borm P.J.A., Kreyling W. (2004). Toxicological hazards of inhaled nanoparticles- Potential implications for drug delivery. J Nanosci Nanotechnol 4(5), 521-531. [This paper gives a brief review on the toxicology of inhaled NP, including general principles and current paradigms to explain the special case of NP in pulmonary toxicology.] Brooking J., Davis S.S., Illum L. (2001). Transport of nanoparticles across the rat nasal mucosa. J Drug Target 9, 267-279. [This paper studied transport of 125I-radiolabelled latex nanoparticles across the nasal mucosa of rats using a range of particle sizes and surface coatings]
Brumfiel G. A. (2003). A little knowledge, Nature 424, 246-248. [This paper firstly advanced and stressed the urgency of nanosafety study] Chen H.W., Su S.F., Chien C.T., Lin W.H., Yu S.L., Chou C.C., ChenJ.J.W., Yang P. C. (2006). Titanium dioxide nanoparticles induce emphysema-like lung injury in mice. Faseb 20,144-153. [This paper investigated the pulmonary toxicity of Titanium dioxide nanoparticles (nanoTiO2) and its molecular pathogenesis] Chen C.Y., Xing G.M, Wang J.X., Zhao Y.L. et al (2005), Multihydroxylated Nanoparticles: Antineoplastic Biological Activity of High-Efficacy and Low-Toxicity, Nano Letters. 4 (10), 1050-1057. [This paper reported the ultrahigh anticancer activities of low-toxic nanoparticles.] Chen Z, Meng H, Xing G.M, Yuan H, Jia G, Chen C. Y, Fang X. H, Ye C, Zhu Z. F, Zhao Y. L. (2006), Acute Toxicological Effects of Copper Nanoparticles in vivo, Toxicology Letters, 163, 109-120. [This paper reported the acute Toxicity of copper metallic nanoparticles in mice, including ADME. LD50, target organs and also size-effects of NPs on the nanotoxicity]
Chen Z., Meng H., Xing G.M., Yuan H., Zhao F., Liu R., Chang X.L., Gao X.Y., Wang T.C., Jia G., Ye C., Chai Z.F., Zhao Y.L. (2008). Age-related differences in pulmonary and cardiovascular responses to SiO2 nanoparticle inhalation: Nanotoxicity shows susceptible population. Environ Sci Technol 42, 89858992. [This article investigated toxicologic sensitivity of nanoparticles in different ages for the first time and pointed out an age-related difference in response to manufactured nanoparticles] Chen Z, Chen H, Meng H, Xing G.M, Gao X.Y., Sun B.Y., Shi X.L., Yuan H, Zhang C.C., Liu R, Zhao F, Zhao Y. L, Fang X.H (2008). Biodistribution and metabolic paths of silica coated CdSeS quantum dots, Toxicology and Applied Pharmacology, 230, 364371, 2008. [This paper studied the biodistribution of quantum dots, and their metabolic pathways, and found three main metabolism paths for quantum dots in vivo.] Delorenzo A.J. (1957). Electron microscopic observations of the olfactory mucosa and olfactory nerve. J Biophys Biochem Cyto 3, 839-850. [This study observed olfactory mucosa and olfactory nerve by electron microscopic.] Elder A., Gelein R., Silva V., Feikert T., Opanashuk L., Carter J., Potter R., Maynard A., Finkelstein J., Oberdorster G. (2006). Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect 114, 1172-1178. [This article concluded that the olfactory neuronal pathway is efficient for translocating inhaled Mn oxide as solid UFPs to the central nervous system and that this can result in inflammatory changes.] Elder A., Gelein R., Silva V., Feikert T., Opanashuk L., Carter J., Potter R., Maynard A., Ito Y., Finkelstein J., Oberdörster G.. (2006). Translocation of inhaled ultrafine manganese oxide particles to the central nervous system, Environ Health Perspect 114, 1172-1178. [This research concluded that the olfactory neuronal pathway is efficient for translocating inhaled Mn oxide as solid ultrafine particles (UFPs) to the central nervous system and that this can result in inflammatory changes] Feng W.Y., Wang B., Zhao Y.L. (2009). Nanotoxicity: from in vivo and in vitro models to health risks. Chapter 11 in: Nanotoxicity of metal oxide nanoparticles in vivo. Publication 191, John Wiley & Sons, Ltd. New York. USA. [Chapter 11 focused on the nanotoxicity of metal oxide nanoparticles in vivo, including the existing sources of nanoparticles in the environment, potential exposure routes and their toxic response via these[F21] routes. This chapter provides some information and guidelines to nanoparticles related occupational health and safety control.] Ferin J., Oberdörster G.., Penney D.P. (1992). Pulmonary retention of ultrafine and fine particles in rats. Am J Respir Cell Mol Biol 6, 535-542. [This paper compared the pulmonary retention of intratracheal instillation. of ultrafine and fine particles in rats]. Fischer H.C., Chan W.C.W. (2007). Nanotoxicity: the growing need for in vivo study. Current Opinion in Biotechnology 18, 565-571. [This paper describe the assumptions and challenges in the nanotoxicity field and provide a rationale for in vivo animal studies to assess nanotoxicity[F22] ]
Chen Z., Meng H., Xing G..M., Chen C.Y., Zhao Y.L.(2007) Toxicological and biological effects of nanomaterials. Int J Nanotechnology 4, 179-196. [This article reviews the recent studies of most recent findings on the toxicological and biological effects of some studied nanomaterials nanotubes, fullerene, metallofullerenes, their derivatives, and metallic nanoparticles[F23] .]
Gao J., Xu B. (2009). Applications of nanomaterials inside cells. Nano Today 4, 37-51. [This paper reported relationship between the prosperities and the applications of nanomaterials inside cells] Gheshlaghi[F24] Z.N., Riazi G.H., Ahmadian S., Ghafari M., Mahinpour R. (2008). Toxicity and interaction of titanium dioxide nanoparticles with microtubule protein. Acta Biochim Biophys Sin 40, 777-782. [This paper investigated the effects of TiO2 Nanoparticles on microtubules polymerization and structure.] Gopalan R.C., Osman I.F., Amani A., De Matas M., Anderson D. (2009). The effect of zinc oxide and titanium dioxide nanoparticles[F25] in the Comet assay with UVA photoactivation of human sperm and lymphocytes. Nanotoxicology 3, 33-39. [This paper evaluated the toxicity of anatase TiO2 and ZnO nanoparticles on peripheral blood lymphocytes and human sperm cells.] Hoet P.H., Bruske-Hohlfeld I., Salata O.W. (2004). Nanoparticles' known and unknown health risks. J Nanobiotechnol 2, 12-27. [This review provides comprehensive analysis of data available on health effects of nanomaterials..] Hoet P., Brüske-Hohlfeld I., Salata O.V. (2004). Nanoparticles known and unknown health risks. J Nanobiotech 2, 12-27. [This review provides comprehensive analysis of data available on health effects of nanomaterials.] Hussain S.M., Hess K.L., Gearhart J.M., Geiss K.T., Schlager J.J. (2005). In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicology in Vitro 19, 975-983. [This paper reported the toxicity of nanoparticles in liver cells in vitro.] ICRP. (1994). Human Respiratory Model for Radiological Protection[F26] . Annals of the ICRP 24:ICRP publication # 66.[This report describes a revision of the model used in ICRP Publication 30 to calculate radiation doses to the respiratory tract of workers resulting from the intake of airborne radionuclides] Jani P., Halbert G.W., Langridge J., Florence A.T. (1989). The uptake and translocation of latex nanospheres and microspheres after Oral-Administration to Rats. Journal of Pharmacy and Pharmacology 41(12), 809-812. [This article showed oral-administered Latex Nanospheres and Microspheres particles were not distributed randomly in the tissues, but were concentrated at the serosal side of the Peyer's patches and could be seen traversing the mesentery lymph vessels towards the lymph nodes[F27] ] Jia G.., Wang H., Yan L., Wang X., Pei R., Yan T., Zhao Y., Guo X. (2005). Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene[F28] . Environ Sci Technol 39, 13781383.[This paper reports carbon nanomaterials with different geometric structures exhibit quite different cytotoxicity and bioactivity in vitro, [F29] although they may not be accurately reflected in the comparative toxicity in vivo.] Karlsson H.L., Cronholm P., Gustafsson J., Moller L. (2008). Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes[F30] . Chem Res Toxicol 21, 1726-1732. [This paper investigated the dose-dependent DNA damage induced by different nanomaterials] Kelly K. L. (2004). Nanotechnology grows up. Science 304, 1732-1734 [This paper expressed the people's concerns about the potential health and environmental impacts of nanotechnology"] Keyhani K., Scherer P.W., Mozell M.M. (1997). A numerical model of nasal odorant transport for the analysis of human olfaction[F31] . J Theor Biol 186, 279-301. [This article investigated the transport and uptake of inspired odorant molecules in the human nasal cavity and determined using an anatomically correct three-dimensional finite element model.] Kreuter J., Ramge P., Petrov V., Hamm S., Gelperina S.E., Engelhardt B. Alyautdin R. von Briesen H. Begley D.J. (2003). Direct evidence that polysorbate-80-coated poly(Butylcyanoacrylate) nanoparticles deliver drugs to the CNS [F32] via specific mechanisms requiring prior binding of drug to the nanoparticles. Pharm Res 20, 409-416. [This study explored more fully the mechanism PBCA nanoparticles delivered drugs to the brain.[F33] ] Kreyling W.G., Semmler M., Erbe F., Mayer P., Takenaka S., Schulz H., Oberdorster G., Ziesenis A. (2002).Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low.[F34] J Toxicol Environ Health A 65, 1513-1530. [This article reviews two important themes of current research into the effects of NSP on the lungs: The potential of NSP to cross the blood-air barrier of the lungs and the entering mechanisms of NSP into different cell types] Kreyling W.G.., Scheuch G. ed. (2000). Clearance of particles deposited in the lungs. Particle-Lung Interactions, ed. Gehr.P., Heyder J., Marcel Dekker: New York. 323-376. [This book describes the clearance of particles deposited in the lungs. particle-lung interactions] Kreyling W.G., Semmler-Behnke M., Möller W.(2006). Ultrafine particle-lung interactions: does size matter? J Aerosol Med 19, 74-83.[This paper reviews the current concept of interactions between insoluble ultrafine particles and biological systems] Lademann J., Weigmann H.J., Rickmeyer C., Barthelmes H., Schaefer H., Mueller G., Sterry W. (1999). Penetration of titanium dioxide microparticles in a sunscreen formulation into the horny layer and the follicular orifice. Skin Pharmacol Appl Skin Physiol 12, 247-256. [This article showed coated titanium dioxide (TiO2) microparticles were commonly used as UV filter substances in commercial sunscreen products. The penetration of these microparticles into the horny layer and the orifice of the hair follicle were investigated]
Li J.J., Zou L., Hartono D., Ong C.N., Bay B.H., Lanry Yung L.Y. (2008). Gold nanoparticles induce oxidative damage in lung fibroblasts in vitro[F35] . Adv Mater 20, 138-142. [This paper investigated the oxidative damage in cells in vitro induced by gold nanoparticles.] Li W., Chen C.Y., Ye C., Wei T.T., Zhao Y.L., Lao F., Chen Z., Meng H., Gao Y.X., Yuan H., Xing G..M., Zhao F., Chai Z.F., Zhang X.J., Yang F.Y., Han D., Tang X.H., Zhang Y.G.. (2008). The translocation of fullerenic nanoparticles into lysosome via the pathway of clathrin-mediated endocytosis, Nanotechnology 19, 145102-145114. [This paper explored the processes of [C60(C(COOH)2)2] nanoparticles across cellular membranes and their intracellular translocation in 3T3 L1 and RH-35 living cells using both microscopic imaging and biological techniques[F36] ] Li W., Zhao F., Chen C.Y., Zhao Y. L. (2009). Cellular biological effects of carbon nanomaterials. Progress in Chemistry 21 (2): 430-435. [This paper summarized the process of internalization , localization in intracellular compartments of carbon nanomaterials (fullerenes , metallofullerenes, carbon nanotubes, and their derivatives) and their succedent effects on the cellular functions] Li Z., Hulderman T., Salmen R., Chapman R., Leonard S.S., Young S.H., Shvedova A., Luster M.I., Simeonova P.P.(2007). Cardiovascular effects of pulmonary exposure to single-wall carbon nanotubes[F37] . Environ Health Perspect 115, 377-382. [This research's findings are of sufficient significance to warrant further studies to evaluate the systemic effects of SWCNT under workplace or environmental exposure paradigms.] Meng H, Chen Z, Xing G.M, Yuan H, Chena C.Y, Zhao F, Zhang C.C., Zhao Y.L (2007). Ultrahigh reactivity provokes nanotoxicity: Explanation of oral toxicity of nano-copper particles[F38] , Toxicology Letters, 175, 102-110. [This work, using the ex vivo mimic experiment together with the in vivo animal experiment, explored how the nanoparticles executed their acute toxicity, and revealed the correlation between the surface reactivity and toxicity. ] Muranishi S., Fujita T., Murakami M., Yamamoto A., 1996. Lymphatic transfer of macromolecules after intrapulmonary administration in the presence or absence of various absorption enhancers in rats. Pharmazie 51, 331-336. [This article assessed biodistribution of inhaled radiolabelled SLN via Lymphatic transfer] Nel A.E., Mädler L., Velegol D., Xia T., Hoek E.M.V., Somasundaran P., Klaessig F., Castranova V., Thompson M. (2009). Understanding biophysicochemical interactions at the nanobio interface. Nat Mat 8, 543-557. [ This Paper reviewed the biophysicochemical interactions of nanoparticles with proteins, membranes, cells, DNA and organelles in the nanoparticle/biological interfaces [F39] and put forward the idea of developing predictive relationships between structure and activity . by probing the various interfaces] Nemmar A., Hoet P.H.M., Vanquickenborne B., Dinsdale D., Thomeer M., Hoylaerts, M.F., Vanbilloen H., Mortelmans L., Nemery B. (2002). Passage of inhaled particles into the blood circulation in humans. Circulation 105, 411-414.[This paper concluded that inhaled 99mTc-labeled ultrafine carbon particles pass
Lam C.W., James J.T., McCluskey R., Hunter R.L. (2004). Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci 77, 126-134. [This article investigated the pulmonary toxicity of single-wall carbon nanotubes in mice after intratracheal instillation in the infant stage of nanotoxicology research]rapidly into the systemic circulation, and this process could account for the well-established, but poorly understood, extrapulmonary effects of air pollution.] Nemmar A., Vanbilloen H., Hoylaerts M.F., Hoet P.H., Verbruggen A., Nemery B. (2001). Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am J Respir Crit Care Med 164(9), 1665-1668. [This article studied the passage of radioactively labeled ultrafine particles after their intratracheal instillation] Oberdörster G., Sharp Z., Atudorei V., Elder A., Gelein R., Kreyling W., Cox C. (2004). Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol 16, 437-445. [This study investigated the translocation of inhaled ultrafine solid particles to regions of the brain and proved a portal of entry into the CNS for solid UFP, circumventing the tight blood-brain barrier.][F40] Oberdörster G.., Ferin J., Lehnert B.E. (1994). Correlation between particle size, in vivo particle persistence, and lung injury. Environ Health Perspect 102, 173-179. [This paper investigated the correlations such as deposition, clearance, retention, and translocation and dissolution of inhaled particles in and to different lung compartments with adverse pulmonary effects using a model involving TiO2 particles of two particle sizes of the same crystalline structure (anatase). Oberdorster G., Sharp Z., Atudorei V., Elder A., Gelein R., Lunts A., Kreyling W., Cox C. (2002). Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A 65, 1531-1543. [This study determined ultrafine elemental carbon particles translocated to the liver and other extrapulmonary organs following inhalation as singlet particles by rats] Oberdörster G., Sharp Z., Atudorei V., Elder A., Gelein R., Kreyling W., Cox C. (2004). Translocation of inhaled ultrafine particles to the brain, Inhal Toxicol 16, 437-445. [This paper concluded from author's studies that inhaled ultrafine carbon particles are to a significant extent translocated to the CNS.] Park E.J., Yi J., Chung K.H., Ryu D.Y., Choi J., Park K. (2008). Oxidative stress and apoptosis induced by titanium in cultured BEAS-2B cells. Toxicol Lett 180, 222-229. [This paper assessed time and dosedependent cytotoxicity of nano-TiO2 on human bronchial epithelial cell line.] Park S.S., Wexler A.S. (2007). Particle deposition in the pulmonary region of the human lung: Multibreath transport and deposition[F41] . J Aerosol Sci 38, 509-519. [This study the deposition and clearance of inhaled particles in the distal pulmonary regions of human airways.] Pekkanen J., Peters A., Hoek G.., Tiittanen P., Brunekreef B., de Hartog J., Heinrich J., Ibald-Mulli A., Kreyling W.G.., Lanki T., Timonen K.L., Vanninen E. (2002). Particulate air pollution and risk of STsegment depression during repeated submaximal exercise tests among subjects with coronary heart disease: the Exposure and Risk Assessment for Fine and Ultrafine Particles in Ambient Air (ULTRA) study. Circulation 106, 933-938. [This article assessed the associations between levels of the 3 main modes of urban aerosol distribution and the occurrence of ST-segment depressions during repeated exercise tests] Pflücker F., Wendel V., Hohenberg H., Gartner E., Will T., Pfeiffer S., Wepf R., Gers-Barla H. (2001). The human stratum corneum layer: An effective barrier against dermal uptake of different forms of topically applied micronised titanium dioxide. Skin Pharmacol Appl Skin Physiol 14, 92-97. [This paper concluded that micronized titanium dioxide is solely deposited on the outermost surface of the stratum corneum and cannot be detected in deeper stratum corneum layers, the human epidermis and dermis.] Rouse J.G., Yang J.Z., Ryman-Rasmussen J.P., Barron A.R., Monteiro-Riviere N.A. (2007). Effects of mechanical flexion on the penetration of fullerene amino acid-derivatized peptide nanoparticles through skin. Nano Lett 7, 155-160. [This article proved fullerene-peptide localization within the intercellular spaces of the stratum granulosum[F42] .] Samet J .M., Dominici F.R., Curriero F.C.D., Coursac I., Zeger S.L. (2000). Fine particulate air pollution and mortality in 20 U. S. cities, 1987-1994. N Engl J Med 343, 1742-1749. [This paper is consistent evidence that the levels of fine particulate matter in the air are associated with the risk of death from all causes and from cardiovascular and respiratory illnesses. These findings strengthen the rationale for controlling the levels of respirable particles in outdoor air.] Schulz J., Hohenberg H., Pflucker F., Gartner E., Will T., Pfeiffer S., Wepf R., Wendel V., Gers-Barlag H., Wittern K.P. (2002). Distribution of sunscreens on skin. Adv Drug Deliv Rev 54, S157-S163.[This investigations using optical and electron microscopy proved that neither surface characteristics, particle size nor shape of the micronized pigments result in any dermal absorption of this substance.] Schulz J., Hohenberg H., Pflucker F., Gartner E., Will T., Pfeiffer S., Wepf R., Wendel V., Gers-Barlag H., Wittern K.P. (2002). Distribution of sunscreens on skin. Adv Drug Deliver Rev 54, S157-S163. [This book investigated the effects of surface characteristics, particle size and shape of the micronised pigments on dermal absorption by using optical and electron microscopy.] Schwart J., Laden F., Zanobetti A.(2002). The concentration-response relation between PM2.5 and daily deaths. Environ Health Perspect 110, 1025-1029. [These reports have used smoothing or spline methods in individual cities and pooled the results across multiple cities to obtain estimates that are more robust.] Service R.F. (2003). "Nanomaterials show signs of toxicity" Science 300, 243. [This paper addressed concerns about nanoparticles' toxicity while the field is still young and exposures limited and stressed the urgency to face this problem before nanotechnology becomes widespread] Shvedova A.A., Castranova V., Kisin E.R, Schwegler-Berry D., Murray A.R., Gandelsman V.Z., Maynard A., Baron P. (2003). Exposure to carbon nanotube material: Assessment of nanotube cytotoxicity using human keratinocytes cells. J Toxicol Environ Health A 1909-1926.[This paper indicated that dermal exposure to unrefined single-wall carbon nanotubes (SWCNT) may lead to dermal toxicity due to accelerated oxidative stress in the skin of exposed workers[F43] .] Singh N., Manshian B., Jenkins G., Griffiths S. M., Williams P. M., Maffeis T.G.G., Wright C. J., Doak S.H. (2009). NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials 30, 3891-3914. [This paper reported the potential DNA damaging of nanomaterials from the release of ROS easily from reacting with cells.] Thomas R., Pisanic II., Jennifer D., Blackwell, Veronica I., Shubayev, Rita R., Finones, Sungho Jin. (2007). Nanotoxicity of iron oxide nanoparticle internalization in growing neurons. Biomaterials 28, 2572-2581. [This paper reported the time and dose dependent neuronal toxicity of iron oxide nanoparticle] Tinkle S.S., Antonini J.M., Rich B.A., Roberts J.R., Salmen R., DePree K. (2003). Skin as a route of exposure and sensitization in chronic beryllium disease. Environ[F44] Health Perspect 111, 1202-1208. [This article used optical scanning laser confocal microscopy and size-selected fluorospheres received data are consistent with development of a hapten-specific, cell-mediated immune response following topical application of beryllium and suggest a mechanistic link between the persistent rate of beryllium worker sensitization and skin exposure to fine and ultrafine beryllium particles.] Tin-Tin-Win-Shwe, Yamamoto S., Ahmed S., Kakeyama M., Kobayashi T., Fujimaki H. (2006). Brain cytokine and chemokine mRNA expression in mice induced by intranasal instillation with ultrafine carbon black. Toxicol Lett 163(2), 153-160.[This paper suggests that the intranasal instillation of ultrafine carbon black (ufCB) may influence the brain immune function[F45] depending on their size.] Videira M.A., Botelho M.F., Santos A.C., Gouveia L.F., De Lima J.J., Almeida A.J. (2002). Lymphatic uptake of pulmonary delivered radiolabelled solid lipid nanoparticles. J Drug Target 10, 607-613. [This article investigated Lymphatic uptake of pulmonary delivered radiolabelled solid lipid nanoparticles.] Wang B., Feng W.Y., Wang M., Shi J.W., Zhang F., Ouyang H., Zhao Y.L., Chai Z.F., Huang Y.Y., Xie Y.N., Wang H.F., Wang J. (2007). Transport of intranasally instilled fine Fe2O3 particles into the brain: Micro-distribution, chemical states, and histopathological observation. Biol Trace Elem Res 118, 233243. [This work describes the initial observation of the transport of intranasally instilled fine ferric oxide (Fe2O3) particles in animal brain, the iron micro-distribution and chemical state in the mice brain on day 14 after intranasal instillation of fine Fe2O3.] Wang B., Feng W.Y., Zhao Y.L., Xing G..M., Chai Z.F., Wang H.F., Jia G.. (2005). Status of study on biological and toxicological effects of nanoscale materials. Sci China Ser B-Chem 48(5), 385-394. (This review paper analyzed and summarized the existing data of the experimental study on the biological activities and adverse effects of nanoscale materials/particles including single wall carbon nanotubes, multi wall carbon nanotubes, titanium oxide and iron powders.) Wang B., Feng W.Y., Wang M., Wang T.C., Gu Y.Q., Zhu M.T., Ouyang H., Shi J.W., Zhang F., Zhao Y.L., Chai Z.F., Wang H.F., Wang J. (2008). Acute toxicological impact of nano- and submicro-scaled zinc oxide powder on healthy adult mice. J Nanopart Res 10, 263-276. [This article evaluated the acute oral toxicity of nano-sized and submicron-sized ZnO nanoparticles, including the distribution of Zn in vivo, serum biochemical, blood viscosity and histopathological damage.] Wang B., Feng W.Y., Wang M., Wang T.C., Gu Y.Q., Zhu M.T., Ouyang H., Shi J.W., Zhang F., Zhao Y. L., Chai Z.F., Wang H.F., Wang J. (2008). Acute toxicological impact of nano- and submicro-scaled zinc oxide powder on healthy adult mice. J Nanopart Res 10(2), 263-276.[This article evaluated the acute oral toxicity of nano-sized and submicron-sized ZnO nanoparticles, including the distribution of Zn in vivo, serum biochemical, blood viscosity and histopathological damage.] Wang J.X., Zhou G.Q., Chen C.Y., Yu H.W., Wang T.C., Ma Y.M., Jia G., Gao Y.X., Li B., Sun J., Li Y.F., Jiao F., Zhao Y.L., Chai Z.F. (2007). Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett 168(2), 176-185. [This article evaluated the acute toxicity of nano-sized TiO2 particles (25 and 80nm), including the changes of serum biochemical parameters and pathology] Wang J.X., Chen C.Y., Liu Y, Jiao F, Li W, Lao F, Li Y.F, Li B, Ge C.C, Zhou G.Q, Gao Y.X, Zhao YL, Chai Z.F. (2008). Potential neurological lesion after nasal instillation of TiO2 nanoparticles in the anatase and rutile crystal phases. Toxicology Letters 183, 72-80. [This work explored the neurotoxicity after nasal instillation of different crystaline of TiO2 nanoparticles, and found that a crystal-type dependent nanotoxicity.] Wang J.X., Liu Y, Jiao F, Lao F, Li W, Gu Y.Q, Li Y.F, Ge C.C, Zhou G.Q, Li B, Zhao YL, Chai Z.F., Chen C.Y (2008). Time-dependent Translocation and Potential Impairment on Central Nervous System by Intranasally Instilled TiO2 Nanoparticles. Toxicology, 254, 82-90.[The paper studied the neurotoxicity of intranasally instilled TiO2 nanoparticles, and found a time-dependent translocation of NPs in brain, which caused impairment on central nervous system.] Warheit D.B., Laurence B.R., Reed K.L., Roach D.H., Reynolds G. A. M., Webb T. R. (2004). Comparative Pulmonary Toxicity Assessment of Single-wall Carbon Nanotubes in Rats. Toxicol Sci 77, 117-125. [This article compared the pulmonary toxicity of single-wall carbon nanotubes in rats after intratracheal instillation in the infant stagy of nanotoxicology research and raised extensive attention in this field] Warheit D.B., Laurence B.R., Reed K.L., Roach D.H., Reynolds G.A.M., Webb T.R. (2004). Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol Sci 77, 117125[This paper evaluated the acute lung toxicity of intratracheally instilled single-wall carbon nanotubes (SWCNT) in rats.] Wichmann H.E., Spix C., Tuch T., Woelke G., Peters A., Heinrich J., Kreyling W.G., Heyder J. (2000). Daily mortality and fine and ultrafine particles in Erfurt, Germany. Part I: Role of particle number and particle mass. Res Rep Health Eff Inst 98, 87-94. [This article investigated the association of mortality not only with ambient particles but also with gaseous pollutants and indicators of sources] Wichmann H.H., Peters A. (2000). Epidemiological evidence of the effects of ultrafine particle exposure. Phil Trans R Soc Lond 358, 2751-2769. [The review investigated the Epidemiological evidence of the effects of ultrafine particle exposure] Wiebert P., Sanchez-Crespo A., Falk R., Philipson K., Lundin A., Larsson S., Moller W., Kreyling W.G., Svartengren M. (2006). No significant translocation of inhaled 35-nm carbon particles to the circulation in humans. Inhal Toxicol 18, 741-747. [This paper investigated translocation of inhaled 35-nm carbon particles to the circulation in humans.] Wojciak-Stothard B., Curtis A., Monaghan W., MacDonald K., Wilkinson C. (1996). Guidance and activation of murine macrophages by nanometric scale topography. Exp Cell Res 223, 426-435. [This article studied the guidance and activation of macrophages from the P388D1 cell line and rat peritoneum by topographic features on a nanometric scale.] Yamago S., Tokuyama H., Nakamura E., Kikuchi K., Kananishi S., Sueki K., Nakahara H., Enomoto S., Ambe F. (1995). In-vivo biological behavior of a water-miscible fullerene-C-14 labeling, absorption, distribution, excretion and acute toxicity. Chem Biol 2, 385-389. [This article assessed the in vivo behavior of fullerenes, including the oral absorption, distribution and excretion of this class of compounds] Yin J.J., Lao F, Fu P, Wamer W, Zhao Y.L, et al (2009). The scavenging of reactive oxygen species and-the potential for cell protection by functionalized fullerene nanomaterials, Biomaterials, 30, 611621.[This study shows that the surface modified fullerene nanoparticles can scavenge reactive oxygen species in cell and hence protect cells from the oxidative damages] Yin J.J., Lao F, Meng J, Fu P, Zhao Y.L, et al (2008), Inhibition of Tumor Growth by Endohedral Metallofullerenol Nanoparticles Optimized as Reactive Oxygen Species Scavenger, Molecular Pharmacology, 74, 1132-1140. [This paper reports that molecular mechanism of the anticancer activity of metallofullerenol nanoparticles] Zhang L.W., Monteiro-Riviere N.A. (2008). Assessment of quantum dot penetration into intact, tapestripped, abraded and flexed rat skin. Skin Pharmacol Phys 21, 166-180. [This article evaluated the penetration of carboxylic coated quantum dot in flow-through diffusion cells with flexed, tape-stripped and abraded rat skin] Zhao Y.L., Nalwa H.S. (2007). Nanotoxicology. American Scientific Publisher. California, USA. [This book is the first monograph about nanotoxicology and is composed of 16 state-of-the-art review chapters on evaluating the safety of nanotechnology, covering the main aspects dealing with the risks and implications of exposure to nanomaterials, especially potential dangers of nanoparticles to human health and safety and the environment. It is a very valuable reference source for nanotechnology industries and public as well as for scientists, students and university professors working in the field of nanotoxicology] Zhao Y.L., Xing G.M., Chai Z.F. (2008). Nanotoxicology: Are Carbon Nanotubes Safe? Nature Nanotechnology 3, 191-192. [This article reviewed the safety of carbon nanotubes using a small number of mice] Zhu M.T., Feng W.Y., Wang B., Wang T.C., Gu Y.Q., Wang M., Wang Y., Ouyang H., Zhao Y.L., Chai Z.F. (2008). Comparative study of pulmonary responses to nano- and submicron-sized ferric oxide in rats. Toxicology 247(2-3), 102-111. [This paper compared lung injury induced by submicron-sized and nanosized Fe2O3 particle intratracheal exposure, including microvascular permeability, cell lysis in lung epitheliums and blood coagulation parameters etc.] Zhu M.T., Feng W.Y., Wang Y., Wang B., Wang M., Ouyang H., Zhao Y.L., Chai Z.F. (2009). Particokinetics and extrapulmonary translocation of intratracheally instilled ferric oxide nanoparticles in rats and the potential health risk assessment. Toxicol Sci 107, 342-351. [This paper investigated particokinetics and extrapulmonary translocation intratracheal-instilled nano-59Fe2O3 particles pass through the alveolar-capillary barrier into systemic circulation within 10 min.] Biographical Sketches
Professor Yuliang Zhao's degrees are in Chemistry (1985, Sichuan Univ.), and in Chemistry/Physics (M.D.1996 & Ph.D. 1999, Tokyo, Japan). He moved to Chinese Academy of Sciences (CAS) from RIKEN (Japan) as a Hundred Elite Professor in 2001. He is the founder of CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, and also the founder of Research Center for Cancer Nanotechnology which is a joint research center from CAS and Tianjin Cancer Hospital. His current researches mainly focus on toxicological effects of nanomaterials, biomedical functions of multifunctional nanoparticles, and nanosurface chemistry for the purposes of enhancing the biomedical functions or reducing the potential toxicity. Dr Zhao`s other research interest includes the theoretical simulation and modeling the dynamic processes of the interplay between nano-systems and bio-systems like membrane or biological channels. Prof. Zhao has published more than 120 peer-reviewed journal articles at International journals, and more than 70 research articles at domestic Chinese journals, one book "Nanotoxicology" in USA (2007), which is the first textbook in the nanotoxicology field. He is also the editor-in-chief of 10 books on biological activities and safety assessment of nanoscale materials, published by China Scientific Press (2009). He serves as editorial board member of 8 international journals, Associate Editors for Biomedical Microdevices (USA), Particle and Fiber Toxicology (UK), and Journal of Nanoscience and Nanotechnology (USA). He has been invited and given more than 110 Invited Lectures at conferences and universities/institutes worldwide. Professor Wei-Yue Feng (born in 1967) obtained a B.S. in Chemistry from Fudan University in 1989, and a Ph.D. from Institute of High Energy Physics, Chinese Academy of Sciences in 1998. Since then, she has been working in the CAS Key Lab for Biomedical Effects of Nanomaterials and Nanosafety of the institute. Her current primary fields of research include nanotoxicology, proteomics and nuclear analytical techniques application. She has published more than sixty related papers at peer-reviewed journals. Now she is a professor and a research group leader at Institute of High Energy Physics, Chinese Academy of Sciences. Professor Bing Wang (born in 1977) obtained a B.S. in Chemistry from Shandong University in 1999, and a Ph.D. from Institute of High Energy Physics, Chinese Academy of Sciences in 2007. Since then, she has been working in the CAS Key Lab for Biomedical Effects of Nanomaterials and Nanosafety of the institute. Her primary fields of research include nanotoxicology and nuclear analytical techniques application. She has published more than ten related papers at peer-reviewed journals. Now she is an assistant professor at Institute of High Energy Physics, Chinese Academy of Sciences. Professor Chunli Bai is Professor and Executive Vice President of the Chinese Academy of Sciences (CAS) and President of the Graduate University of CAS with more than 32,000 students, Director of Division of Chemistry and member of Executive Committee of the Presidium. He graduated from the Department of Chemistry, Peking University in 1978 and received his MS and Ph.D. degrees from CAS Institute of Chemistry in 1981 and 1985, respectively. During 1985-1987, he was at Caltech, the US for advanced study, conducting research work in the field of physical chemistry as a post-doctorate associate and visiting scholar. After his return home in 1987, he continued his research at CAS Institute of Chemistry. From 1991 to 1992, he was a visiting professor at Tohoku University in Japan. Prof. Bai has a long list of scientific publications and has won more than twenty prestigious awards and prizes for his academic achievements. Because of his meritorious service, He was elected a member of CAS and a fellow of the Academy of Sciences for the Developing World (TWAS) in 1997. He is also foreign associate of the US National Academy of Sciences, fellow of the Royal Society of Chemistry, foreign member of the Mongolian National Academy of Sciences and honorary doctor or honorary professor in several universities abroad. Prof. Bai now serves as the chief scientist for the National Steering Committee for Nanoscience. In his social activities, he is president of Chinese Chemical Society, honorary president of Chinese Society of Micro-Nano Technology (CSMNT), The Vice-President of the China Association for Science and Technology, Vice-President of TWAS, member of Bureau and Executive Committee of IUPAC. President-Elect of Federation of Asian Chemical Societies. He is the member of the International Editorial Adversary Board of JACS, Angewandte Chemie Advanced Materials and Chemical Physics Letters.