- ICON Virtual Journal of Nanotechnology Environment, Health and Safety (VJ-NanoEHS) compiles papers in peer-reviewed journals that address EHS issues in nanotechnology.
- ISI - Web of Science Database contains peer reviewed journals, their references and citations. It also has very useful tools such as 'find related papers' that searches for papers sharing the same references as the entry you're looking at. This is the database behind the compilation of the journal citation factors.
- Knovel - Online Handbook Collection and Database is an extensive collection of handbooks and tables.
- PROLA - the Physical Review Online Archive searches Physical Review journals.
- Rubber Bible Online is a physical chemistry handbook which contains tables of physical and chemical data.
- Spin AIP Scitation searches related journals.
- Web of Science by ISI generates the impact factors (see journals below).
- Virtual Journal of Nanotechnlogy collects nanotech related papers from non-nano specialized journals.
- Derwent Patent database
Overview of the nanotechnology related journals and their impact factors (2007 values):
Nanotechnology Related Journals
Name
Web
Comments
ACS Nano
N/A
1936-0851
general nanotech journal
Advanced Functional Materials
7.5
1616-301X
?
Advanced Materials
8.2
0935-9648
?
American Journal of Physics (AJP)
0.9
0002-9505
?
Applied Physics A: Materials Science & Processing
1.9
0947-8396
?
Applied Physics Letters (APL)
3.6
?
?
AZojono - Journal of Nanotechnology Online
N/A
?
Free access journal
Chemical Reviews
22.8
0009-2665
?
Current Nanoscience
2.8
1573-4137
Reviews and original research reports
Fullerenes, Nanotubes, and Carbon Nanostructures
0.5
1536-383x
all areas of fullerene research
IEEE Transactions on Nanotechnology
2.1
1536-125X
physical basis and engineering applications of nanotechnology
International Journal of Nanomedicine
N/A
1176-9114
?
International Journal of Nanoscience
N/A
0219-581X
New nanotech journal (Feb 2002)
Japanese Journal of Applied Physics
1.2
1347-4065
?
Journal of Applied Physics
2.2
?
?
Journal of Biomedical Nanotechnology
[]
N/A
?
JBN is a peer-reviewed multidisciplinary journal providing broad coverage in all research areas focused on the applications of nanotechnology in medicine, drug delivery systems, infectious disease, biomedical sciences, biotechnology, and all other related fields of life sciences.
Journal of Experimental Nanoscience
N/A
1745-8080
New nanotech journal(March 2006)
Journal Of Microlithography Microfabrication And Microsystems
N/A
1537-1646
Journal of Micromechanics and Microengineering
1.9
0960-1317
?
Journal of Nano Research
N/A
1661-9897
Journal of Nanomaterials
N/A
?
science and applications of nanoscale and nanostructured materials
Journal of Nanoparticle Research
2.3
1388-0764
?
Journal of Nanoscience and Nanotechnology
2.0
?
JNN is a multidisciplinary peer-reviewed journal covering fundamental and applied research in all disciplines of science, engineering and medicine. JNN publishes all aspects of nanoscale science and technology dealing with materials synthesis, processing, nanofabrication, nanoprobes, spectroscopy, properties, biological systems, nanostructures, theory and computation, nanoelectronics, nano-optics, nano-mechanics, nanodevices, nanobiotechnology, nanomedicine, nanotoxicology.
Journal of Physical Chemistry A
2.9
?
?
Journal of Physical Chemistry B
4.1
?
?
Journal of Physical Chemistry C
N/A
?
Nanomaterials and Interfaces, Nanoparticles and Nanostructures, Surfaces, Interfaces, Catalysis, Electron Transport, Optical and Electronic Devices, Energy Conversion and Storage
Journal of the American Chemical Society (JACS)
7.9
?
Multidisciplinary chemistry journal
Journal of Vacuum Science & Technology A (JVSTA)
1.3
?
Vacuum, Surfaces, Films
Journal of Vacuum Science & Technology B (JVSTB)
1.4
?
Microelectronics and Nanometer Structures: Processing, Measurement, and Phenomena
Langmuir
4.0
?
Research in the fields of colloids, surfaces, and interfaces
Micron
1.7
?
Journal for Microscopy
Materials Chemistry and Physics
1.9
0254-0584
materials science, including nanomaterials and opto electronics
Materials Science and Engineering: C
1.3
0928-4931
Biomimetic and Supramolecular Systems
Materials Science and Engineering: R: Reports
17.7
0927-796X
Invited review papers covering the full spectrum of materials science and engineering
Materials Today
N/A
1369-7021
materials science and technology
Microfluidics and Nanofluidics
2.2
1613-4982
all aspects of microfluidics, nanofluidics, and lab-on-a-chip science and technology
Microscopy Research and Technique
1.6
?
?
Nano
N/A
1793-2920
New nanotech journal (July 2006)
Nano Letters
9.6
?
General nanotechnology journal
Nanomedicine
2.8
?
?
Nanopages
N/A
1787-4033
Since sept 2006.
Nano Research
N/A
?
First issue july 2008
Nano Research Letters
wait
1931-7573
articles with open access
Nanotechnology
3.3
?
Journal specializing in nanotechnology
NanoToday
N/A
?
Is this peer reviewed or more a news/reviews journal?
Nature
31.434
?
One of the major journals in science
Nature Biotechnology
22.8
?
advances in life sciences
Nature Materials
19.8
?
covers a range of topics within materials science
Nature Methods
15.5
?
tried-and-tested techniques in the life sciences and related area of chemistry
Nature Nanotechnology
14.9
?
mix of news, reviews, and research papers
Nanotoxicology
N/A
1743-5404
Research relating to the potential for human and environmental exposure, hazard and risk associated with the use and development of nano-structured materials
Open Nanoscience Journal
N/A
1874-1401
Open access journal with research articles, reviews and letters.
Physical Review Letters (PRL)
6.9
?
One of the top physics journals
PLoS Biology
13.5
1544-9173
Peer reviewed open access bio journal
PLoS ONE
N/A
?
Peer reviewed open access science journal
Proceedings of the National Academy of Sciences(PNAS)
10.2
?
multidisciplinary scientific serial: biological, physical, and social sciences.
Recent Patents on Nanotechnology
N/A
1872-2105
?
Science
26.4
?
One of the major journals in science
Solid-State Electronics
1.3
?
?
Small Journal
6.4
1613-6810
New nanotech journal
Smart Materials and Structures
1.5
0964-1726
since 1992
Thin Solid Films
1.7
0040-6090
Thin-film synthesis, characterization, and applications.
Ultramicroscopy
2.0
?
Microscopy related research.
Virtual Journal of Nanotechnlogy
N/A
1553-9644
Collecting nanotech related papers from non-nano spcialized journals
- Impact factors are only guides to how much a papers is referenced in the years just after publication.
- Please add comments about the journals and update impact factors!
- The Physics and Astronomy Classification Scheme PACS2006 and Nanoscale Science and Technology - Collection of Applicable Terms from PACS 2006
******************************************************************
Identification of key hazard properties
Size is the general reason why nanoparticles have become a matter of discussion and concern.-The very small dimensions of nanoparticles increases the specific surface area in relation to mass, which again means that even small amounts of nanoparticles have a great surface area on which reactions could happen.--If a reaction with chemical or biological components of an organism leads to a toxic response, this response would be enhanced for nanoparticles. This enhancement of the inherent toxicity is seen as the main reason why smaller particles are generally more biologically active and toxic that larger particles of the same material [52].---Size can cause specific toxic response if for instance nanoparticles will bind to proteins and thereby change their form and activity, leading to inhibition or change in one or more specific reactions in the body [53][F4].
Besides the increased reactivity, the small size of the nanoparticles also means that they can easier be taken up by cells and that they are taken up and distributed faster in organism compared to their larger counterparts [54], [55].--Due to physical and chemical surface properties all nanoparticles are expected to absorb to larger molecules after uptake in an organism via a given route of uptake [56].
Some nanoparticles such as fullerene derivates are developed specifically with the intention of pharmacological applications because of their ability of being taken up and distributed fast in the human body, even in areas which are normally hard to reach – such as the brain tissue [57]. Fast uptake and distribution can also be interpreted as a warning about possible toxicity, however this need not always be the case [58]. Some nanoparticles are developed with the intension of being toxic[F5] for instance with the purpose of killing bacteria or cancer cells [59], and in such cases toxicity can unintentionally lead to adverse effects on humans or the environment.
Due to the lack of knowledge and lack of studies, the toxicity of nanoparticles is often discussed on the basis of ultra fine particles (UFPs), asbestos, and quartz, which due to their size could in theory fall under the definition of nanotechnology [60], [61].--An estimation of the toxicity of nanoparticles could also be made on the basis of the chemical composition, which is done for instance in the USA, where safety data sheets for the most nanomaterials report the properties and precautions related to the bulk material [62].--Within such an approach lies the assumption that it is either the chemical composition or the size that is determining for the toxicity. However, many scientific experts agree that that the toxicity of nanoparticles cannot and should not be predicted on the basis of the toxicity of the bulk material alone[F6] [63], [64].
The increased surface area-to-mass ratio means that nanoparticles could potentially be more toxic per mass than larger particles (assuming that we are talking about bulk material and not suspensions), which means that the dose-response relationship will be different for nanoparticles compared to their larger counterparts for the same material. This aspect is especially problematic in connection with toxicological and ecotoxicological experiments, since conventional toxicology correlates effects with the given mass of a substance [65], [66].
Inhalation studies on rodents have found that ultrafine particles of titanium dioxide causes larger lung damage in rodents compared to larger fine particles for the same amount of the substance. However, it turned out that ultra fine- and fine particles cause the same response, if the dose was estimated as surface area instead of as mass [67].
This indicates that surface area might be a better parameter for estimating toxicity than concentration, when comparing different sizes of nanoparticles with the same chemical composition5. Besides surface area, the number of particles has been pointed out as a key parameter that should be used instead of concentration [68].
Although comparison of ultrafine particles, fine particles, and even nanoparticles of the same substance in a laboratory setting might be relevant, it is questionable whether or not general analogies can be made between the toxicity of ultrafine particles from anthropogenic sources (such as cooking, combustion, wood-burning stoves, etc.) and nanoparticles, since the chemical composition and structure of ultrafine particles is very heterogeneous when compared to nanoparticles which will often consists of specific homogeneous particles [69].
From a chemical viewpoint nanoparticles can consist of transition metals, metal oxides, carbon structures and in principle any other material, and hence the toxicity is bound to vary as a results of that, which again makes in impossible to classify nanoparticles according to their toxicity based on size alone [70].[F7]
Finally, the structure of nanoparticles has been shown to have a profound influence on the toxicity of nanoparticles. In a study comparing the cytotoxicity of different kinds of carbon-based nanomaterials concluded that single walled carbon nanotubes was more toxic that multi walled carbon nanotubes which again was more toxic C60[F8] [71].
Hazard identification
In order to complete a hazard identification of nanomaterials, the following is ideally required
- ecotoxicological studies
- data about toxic effects
- information on physical-chemical properties
- Solubility
- Sorption
- biodegradability
- accumulation
- and all likely depending on the specific size and detailed composition of the nanoparticles
In addition to the physical-chemical properties normally considered in relation to chemical substances, the physical-chemical properties of nanomaterials is dependent on a number of additional factors such as size, structure, shape, and surface area. Opinions on, which of these factors are important differ among scientists, and the identification of key properties is a key gap of our current knowledge [72], [73], [74].
There is little doubt that the physical-chemical properties normally required when doing a hazard identification of chemical substances are not representative for nanomaterials, however there is at current no alternative methods. In the following key issues in regards to determining the destiny and distribution of nanoparticles in the environment will be discussed, however the focus will primarily be on fullerenes C60.
Solubility
Solubility in water is a key factor in the estimation of the environmental effects of a given substance since it is often via contact with water that effects-, or transformation, and distribution processes occur such as for instance bioaccumulation.---Solubility of a given substance can be estimated from its structure and reactive groups. For instance, Fullerenes consist of carbon atoms, which results in a very hydrophobic molecule, which cannot easily be dissolved in water. Fortner et al. [75] have estimated the solubility of individual C60 in polar solvents such as water to be 10-9 mg/L. When C60 gets in contact with water, aggregates are formed in the size range between 5-500 nm with a greater solubility of up to 100 mg/L, which is 11 orders of magnitude greater than the estimated molecular solubility. This can, however, only be obtained by fast and long-term stirring in up to two months. Aggregates of C60 can be formed at pH between 3.75 and 10.25 and hence also by pH-values relevant to the environment [75].
As mentioned the solubility is affected by the formation of C60 aggregates, which can lead to changes in toxicity [75]. C60 will neither behave as molecules nor as colloids in aqueous systems, but rather as a mixture of the two [81], [82].
Aggregates form reactive free radicals, which can cause harm to cell membranes, while free[F9] C60 kept from aggregation by coatings do not form free radical [76]. Gharbi et al. [77] point to the accessibility of double bonds in the C60 molecule as an important precondition for its interactions with other biological molecules.--- The solubility of C60 is less in salt water, and according to Zhu et al. [78] only 22.5 mg/L can be dissolved in 35 ‰ sea water. Fortner et al. [79] have found that aggregate precipitates from the solution in both salt water and groundwater with an ionic strength above 0.1 I, but aggregates would be stable in surface- and groundwater, which typically have an ionic strength below 0.5 I.--The solubility of C60 can be increased to about 13,000-100,000 mg/L by chemically modifying the C60–molecule with polar functional groups such as hydroxyl [80]. The solubility can furthermore be increased by the use of sonication or the use of none-polar solvents.
The chemical properties of individual C60 such as the log octanol-water partitioning coefficient (log Kow) and solubility are not appropriate in regard to estimating the behavior of aggregates of C60. Instead properties such as size and surface chemistry should be applied a key parameters [83]18.-Just as the number of nanomaterials and the number of nanoparticles differ greatly so does the solubility of the nanoparticles. For instance carbon nanotubes have been reported to completely insoluble in water [84]. It should be underlined that which method is used to solute nanoparticles is vital when performing and interpreting environmental and toxicological tests.
Evaporation
Information about evaporation of C60 from aqueous suspensions has so far not been reported in the literature and since the same goes for vapor pressure and Henry’s constant, evaporation cannot be estimated for the time being. Fullerenes are not considered to evaporate – neither from aqueous suspension or solvents – since the suspension of C60 using solvents still entails C60 after evaporation of the solvent [85], [86].
Sorption
According to Oberdorster et al. [87] nanoparticles will have a tendency to sorb to sediments and soil particles and hence be immobile because of the great surface area when compared to mass. Size alone will furthermore have the effect that the transport of nanoparticles will be dominated by diffusion rather than van der Waal forces and London forces, which increases transport to surfaces, but it is not always that collision with surfaces will lead to sorption [88].
For C60 and carbon nanotubes the chemical structure will furthermore result in great sorption to organic matter and hence little mobility since these substances consists of carbon. However, a study by Lecoanet et al. [89]45 found that both C60 and carbon nanotubes are able to migrate [F10]through porous medium analogous to a sandy groundwater aquifer and that C60 in general is transported with lower velocity when compared to single walled carbon nanotubes, fullerol, and surface modified C60. The study further illustrates that modification of “on the way to - or after “- the outlet into the environment can profoundly influence mobility. Reactions with naturally occurring enzymes [90], electrolytes or humid acid can for instance bind to the surface and make thereby increase mobility [91], just as degradation by UV-light or microorganisms could potentially results in modified C60 with increased mobility [92]45.
Degradability
Most nanomaterials are likely to be inert [93], which could be due to the applications of nanomaterials and products, which is often manufactured with the purpose of being durable and hard-wearing. Investigations made so far have however showed that fullerene might be biological degradable whereas carbon nanotubes are consider biologically non-degradable [94], [95]. According to the structure of fullerenes, which consists of carbon only, it is possible that microorganisms can use carbon as an energy source, such as it happens for instance with other carbonaceous substances.--Fullerenes have been found to inhibit the growth of commonly occurring soil- and water bacteria [96], [97] , which indicates that toxicity can hinder degradability. It is, however, possible that biodegradation can be performed by microorganisms other than the tested microorganisms, or that the microorganism adapt after long-term exposure[F11]. Besides that can be degraded by UV-light and O [98]. UV-radiation of C60 dissolved in hexane, lead to a partly or complete split-up of the fullerene structure depending on concentration [99].
Bioaccumulation
Carbon based nanoparticles are lipophilic which means that they can react with and penetrate different kinds of cell membranes [100]. Nanomaterials with low solubility (such as) could potentially accumulate in biological organisms [101] , however to the best of our knowledge no studies have been performed investigating this in the environment. Biokinetic studies with in rats result in very little excretion which indicates an accumulation in the organisms [102]. Fortner et al. [103] estimates that it is likely that nanoparticles can move up through the food chain via sediment consuming organisms, which is confirmed by unpublished studies performed at Rice University, U.S[F12]. [104] Uptake in bacteria, which form the basis for many ecosystems, is also seen as a potential entrance to whole food chains [105
Interactions in the Environment
Nanoparticles can be used to enhance the bioavailability of other chemical substances so that they are easily degradable or harmful substances can be transported to vulnerable ecosystems [113].[F13]--Besides the toxicity of the nanoparticles itself, it is furthermore unclear whether nanoparticles increases the bioavailability or toxicity of other xenobiotics in the environment or other substances in the human body. Nanoparticles such as C60[F14] have many potential uses in for instance in medicine because of their ability to transport drugs to parts of the body which are normally hard to reach. However, this property is exactly what also may be the source to adverse toxic effects [114]. Furthermore research is being done into the application of nanoparticles for spreading of contaminants already in the environment. This is being pursued in order to increase the bioavailability for degradation of microorganisms [115]54, however it may also lead to increase uptake and increased toxicity of contaminants in plants and animals,[F15] but to the best of our knowledge, no scientific information is available that supports this [116], [117
*********************************************************************
Nanomaterials Safety Guidelines
The study of nanoparticles and uncovering their potential to be incorporated in technology has evolved rapidly in the last two decades. Many of these nanoparticles are being added in merchandises such as wrinkle and stain resistant fabrics, sunscreen, glare-resistance eye glasses, medical uses and much more. Although this evolution of nanotechnology is vastly improving our current materials, the hazardous properties of these nanoparticles and their implication within workplace safety is long overdue and is now stated to be investigated. Concordia University Environmental Health & Safety (EHS) has developed this following document to provide guidelines concerning the work with nanoparticles within its laboratory facilities. These guidelines provide information about the properties of commonly used nanoparticles/nanomaterials, their health and safety hazards and ways to protect oneself from potential laboratory exposures. Emergency procedures for dealing with accidental spills or nanoparticles exposure are also included.
WARNING: Since nanotechnology is an emerging field, many of the hazardous effects are not completely understood with many nanoparticles. Since these materials are relatively new, they are to be considered toxic and handled cautiously [a "precaution principle" as stated by the European Trade Union Confederation (ETUC)] until adequate amount of data on the hazards of these nanomaterials has been collected for health and environment safety information. 1) Definitions
Nanoparticles can occur naturally in nature (e.g. volcanic eruptions, ocean spray, dust volatilization and etc.), occur by accident (e.g. as side product from a reaction) or can be purposefully engineered.
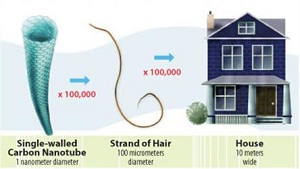
They are defined as material purposefully produced with at least one dimension in the 1 - 100 nanometer range (as stated by the American Society for Testing and Materials' (ASTM) Committee on Nanotechnology). These materials are investigated since they have unique properties compared to their bulk and atomic counterparts. These novel properties in engineered nanoparticles are widely used and consumer's products containing engineered nanomaterials are quickly rising. However, with these unique properties and characteristics (such as size), it is possible that these materials have an increased toxicity compared to their counterparts (the bulk and atomic scale) (Fig. 1).
Figure 1: Size comparison of nanoparticles (Picture obtained from https://www.niehs.nih.gov/health/topics/agents/sya-nano/)
b) Ultra-fine particles
Often referred as nanometer-diameter particles that are not intentionally produced (less than 100 nm size), such as naturally airborne particles or incidental products of processes involving combustion (e.g.: carbon black, smoke, welding fumes).
c) Engineered nanomaterial
Intentionally manufactured material, containing particles (unbound state, aggregate or agglomerate) and where, for 50% or more of the particles in the number size distribution, one or more external dimensions is in the size range 1-100 nm.
d) Nanopowder A mass of dry nanoparticles.
e) Nanoaerosol
A collection of nanoparticles suspended in a gas.
f) Nanofibre A nano-object with two similar external dimensions in the nanoscale and the third dimension significantly larger (Fig. 2). A nanofibre can be flexible or rigid. The two similar external dimensions are considered to differ in size by less than three times and the significantly larger external dimension is considered to differ from the other two by more than three times. The largest external dimension is not necessarily in the nanoscale. If the nanofibre has a length greater than 5 m, a width less than 3 m and a length to width ratio (aspect ratio) greater than 3:1, it is called a World Health Organization (WHO) nanofibre.
g) Nanoplatelet
A nanoparticle having a "platelet" morphology (a minute flattened body) that presents only one dimension in the nanoscale (meaning they have a very thin but wide aspect ratio) (Fig. 2).
Figure 2: Carbon nanofibers (left) and silver nanoplatelets (above)
h) High Aspect Ratio Nanoparticles (HARNs) Particles with one or two dimensions in the nanoscale that are much smaller than the others. Nanofibres and nanoplatelets are considered as HARNs.
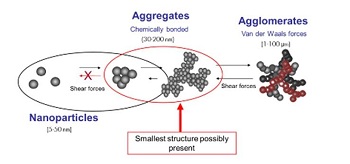
i) Agglomerate-A group of nanoparticles held together by relatively weak forces (van der Waals, electrostatic or surface tension) (Fig. 3)
Figure 3: Nanoparticle aggregates and agglomerates (Picture obtained from http://www.teknoscienze.com/Articles/HPC-Today-Nanotechnology-and-Sun-Care-ARisk-Review.aspx)
j) Aggregate
A heterogeneous particle in which the various components are held together by relatively strong forces not easily broken apart (Fig. 3).
2) Types of Engineered Nanoparticles
a) Carbon-Based Particles
These are nanoparticles that are commonly composed of carbon. Their shapes may be in the form of spheres, ellipsoids as well as tubes. The spheres are known as fullerenes and the tubes are known as carbon nanotubes (Fig. 4).
Fullerene[F16]
Carbon Nanotubes
Figure 4: Carbon-based engineered nanoparticles (Picture obtained from - http://www.physics.ox.ac.uk/nanotech/research/nanotubes/index.html)
b) Metal-Based Particles
These nanoparticles include metal oxides (e.g. TiO2, ZnO, CeO2), quantum dots (semiconductor devices with chemical composition of CdSe or ZnS) and zero-valence metals (e.g. zero-valence Fe, colloidal silver and gold). They consist of closely packed metals with particulate sizes of a few nanometers to a 100 nm in diameter. These particles also have size sensitive optical properties (Fig. 5).
Metal Based Nanoparticle (Gold)
Quantum Dot (CdSe)[F17]
Quantom Dot Variables[F18]
Figure 5:
Metal-based engineered nanoparticles
EHS-DOC-035 v.2 4 / 30-(Picture obtained from (Gold) - http://news.brown.edu/pressreleases/2013/10/nanogold (Quantum Dot) http://portal.uni-freiburg.de/ak-labahn/forschung/fluoreszenz)
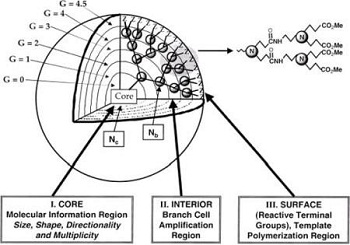
These nanoparticles are synthesized polymers with many side branches. Different functional groups are on the surface of these dendrimers and are used for different function for applications. In addition, they have cavities in the inside that can host other types of molecules (Fig. 6).
Figure 6: Dendrimer engineered nanoparticles
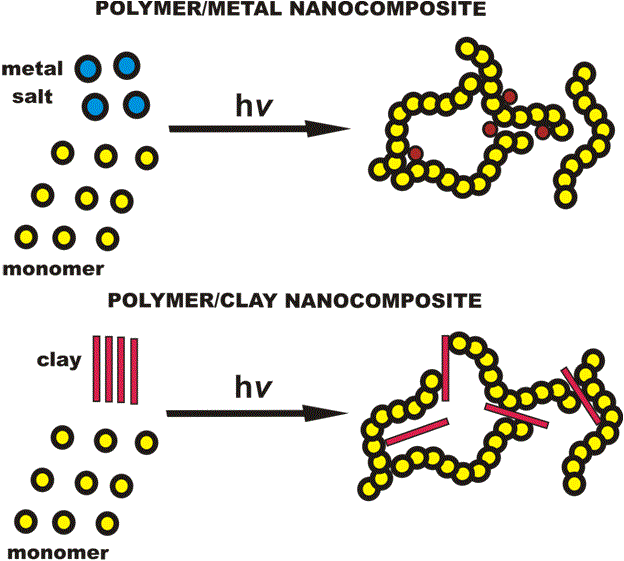
d) Composites
These are nanoparticles combined with other nano or bulk materials. Some examples include using nanoparticles in a wide variety of merchandises to improve their current properties. Nanoclays are another example in particular (including mineral silicates, montmorillonite, bentonite, kaolinite, hectorite and hallosite) (Fig. 7).
Figure 7: Nanocomposite materials (Picture obtained from [F19] http://web.itu.edu.tr/~yusuf/index-1.htm)
3) Properties
a) Names
1-dimensional nanomaterials (they are extended in the other two dimensions) are known as layers. e.g. Thin film or surface coatings 2-dimensional nanomaterials (they are extended in one dimension only) are known as wires. e.g. Nanowires or carbon nanotubes 3-dimensional nanomaterials (all three dimensions are in the nanoscale) are known as nanoprecipitates. e.g. Nanoparticles or quantum dots
Table 1: CAS
Number of Common Nanoparticles--Nanoparticles Carbon Nanotubes Fullerenes TiO2 ZnO CeO2 Quantum Dots (CdSe) Quantum Dots (CdS) Quantum Dots (CdTe) Zero Valent Iron Silver Nanoparticles Gold Nanoparticle
EHS-DOC-035 v.2
CAS # 308068-56-6 99685-96-8 13463-67-7* 1314-13-2* 1306-38-3* 1306-24-7 1306-23-6 106627-54-7 7439-89-6 7440-22-4* 7440-57-5*
6 / 30
Note - * There is no exact CAS # for these nanoparticles, rather it is for their bulk material
Table 2: Common uses of Nanoparticles
Engineered Nanoparticles Applications
Carbonaceous Compounds Carbon Nanotubes and their Derivatives Electronics, computers, plastics, catalysts, batteries, conductive coatings, supercapacitors, water purification systems, orthopedic implants, aircraft, sporting goods, car parts, concrete, ceramics, solar cells, textiles Removal of organometallic compounds, cancer treatment, cosmetics, magnetic resonance imaging, X-ray contrasting agent, anti-viral therapy Metal Oxides TiO2 Sunscreen lotions, cosmetics, skin care products, solar cells, food colorant, clothing, sporting goods, paints, cement, windows, electronic coatings, bioremediation Skin care products, bottle coatings, gas purification, contaminant sensors Combustion catalyst in diesel fuels, solar cells, oxygen pumps, coatings, electronics, glass/ceramics, ophthalmic lenses Semi-Conductor Devices Quantum dots Medical imaging, targeted therapeutics, solar cells, photovoltaic cells, security links, telecommunications Zero-Valence Metals Zero-valent iron Remediation of water, sediments and soils to remove nitrates, detoxification of organochlorine pesticides and polychlorinated biphenyls
ZnO CeO2
Nanoparticulate silver
Textiles (e.g., socks, shirts, pants), disinfectant sprays, deodorants, laundry soaps, wound dressings, air filters, toothpaste, baby products (milk bottles, teethers), cosmetics, medical instruments, hardware (computer, mobile phones), food storage containers, cooking utensils, food additive/supplements, appliances (hair dryers, vacuum cleaners, washing machines, refrigerators), coatings/paints Tumor therapy, flexible conducting inks or films, catalyst, cosmetics, pregnancy tests, anti-microbial coatings Polymers
Colloidal elemental gold
Drug delivery, tumor treatment, manufacture of macrocapsules, nanolatex, coloured glasses, chemical sensors, modified electrodes
b) Additional Physical Properties
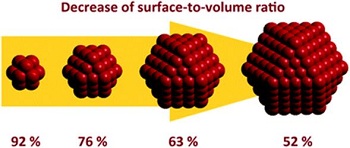
Relative surface area and quantum effects are two important properties observed in nanoparticles compared to those in the bulk and atomic scale. These factors have the tendency to change/enhance reactivity, strength, optical and electrical properties of the nanoparticles compared to their bulk counterpart. 1) Relative Surface Area The decrease of material size increases the number of surface atoms (or molecules) compared to the rest of the material (meaning atoms [or molecules] inside of the material)[F22] (Fig. 8). For example, a nanoparticle with a size of 30 nm will have 5% of its atoms on the surface, 10 nm will have 20% of its atoms on the surface and a 3 nm particle will have 50% of its atoms on the surface. Therefore, smaller particles have a larger surface area per units of mass compared to their bulk material[F23]. This means that an increase of efficiency can be observed with the nanoparticle compared to their bulk counterpart when using the same mass.
Figure 8: Surface-to-volume ratio of nanoparticles (Picture obtained from: Bäumer et al., Phys. Chem. Chem. Phys., 2011, 13, 19270-19284)
2)
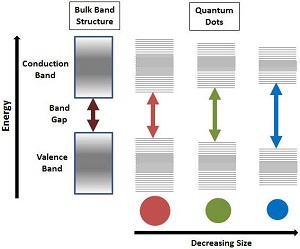
Quantum Effects When reducing the size of materials to the nanoscale, quantum effects begin to dominate (Fig. 9). These effects can change or enhance strength, optical and electrical properties. An example of this is the reduced defects on the surface for the nanoparticle which increases the strength of the nano-crystalline structure. Another example is the band gap change of bulk semiconductor relative to a quantum dot where fluorescence can be observed and controlled by the size of the quantum dot.--Figure 9: Quantum effects of size reduction of nanoparticles (Picture obtained from - http://www.sigmaaldrich.com/materials-science/nanomaterials/quantum-dots.html)
Due to the differential changes in size and quantum effect properties of nanoparticles, the information available is limited concerning their potential threat on human health or on the environment. However, this does not negate the responsibility of protecting oneself from nanoparticles. When working with unknown materials, precautions must be taken in order to establish possible threats by examining potential dangerous scenarios and preventing them from happening. This includes toxicity issues, chemical dangers, fires, explosions, dusts inhalation, electrostatic, size, shape and concentration. Some examples include: Change of property based on size - it has been observed that >80 nm size aluminum nanoparticle dust is very inert compared to its smaller size counterpart (< 80 nm), which is very reactive and explosive. Chemical toxicity quantum dots are generally made with toxic ions [Cd2+ or Hg2+] and the quantum dots can dissociate into their ion state in the body or environment.
a) Legislation
There is currently no nanomaterial/nanoparticle-specific legislation in Quebec or Canada. In Quebec, the Part 1 of the Regulation Respecting Occupational Health and Safety provides permissible exposure limits for many chemicals of the same composition than nanoparticles. However, it does not take into consideration the particle size or the possibility of a different toxicity according to the granulometry of the specific chemical. Health Canada and Environment Canada are currently studying a possible distinct legislation concerning the manufacturing, import and export of nanomaterials, including their use and their environmental management. The only current obligation for manufacturers or importers is to provide restricted information to Environment Canada or Health Canada. Other countries (USA and EU) are now considering nanomaterials as new chemical substances. Since 2008, the EPA requires certain nanomaterials (fullerenes, quantum dots, carbon nanotubes) to go through its New Chemical Review process. However, no official occupational exposure limit values (OELs) specific to nanomaterials have been set in the USA or in the EU level. For certain nanomaterials, industry, research organisms and agencies have suggested either specific OELs (Table 3). Some companies and research institutes have also proposed an OEL for singlewall and/or multiwall carbon nanotubes (SWCNT & MWCNTs) (Bayer, Nanocyl and NIOSH). A threshold value for Carbon Nanotubes has also been set in Switzerland in 2011 by the Swiss National Accident Insurance Fund at 0.01 fibres/mL. In any event, as a minimum, it is necessary to ensure compliance with any existing generic threshold limit values, such as general dust limit-values for alveolar and respirable dust fractions, irrespective of the sources contributing to these fractions.
Table 3: Suggested OELs
Product Carbon nanotubes (SWCNT and MWCNT) Fibrous nanomaterials TiO2 (10-100 nm) TiO2 (21 nm) Fullerenes Insoluble nanomaterials (<100 nm) Soluble nanomaterials *TLV: threshold limit value Proposed Limit 1 g/m3 0.1 fibre/mL 0.3 mg/m3 1.2 mg/m3 0.8 mg/m3 20,000 part/mL 0.5 X TLV* Organism NIOSH Safe Work Australia NIOSH AIST AIST Dutch Social Partners Dutch Social Partners
The risk of exposure strongly depends on the state of the nanoparticle. The potential risks for health are usually limited when working with nanomaterials incorporated in a solid matrix, if this one is not grinded, cut or physically transformed. When handling nanoparticles in solution (slurry), risks are usually associated with the nanoaerosol formation arising from mixing, stirring or sonication. The biggest exposure risks are coming when dry (louse) nanoparticles powder are being handled. Nanoparticles are known to travel great distances in air because of their increased mobility. In preventing exposure, there are three main routes that nanoparticles can enter the human body: inhalation into the pulmonary system; absorption through the dermal system; ingestion through the gastrointestinal system[F24]. It must be highlighted that inhalation exposure is of the greatest concern with regard to the effects of particulate nanomaterials on occupational health, and special attention is being given to studying
impacts on the respiratory system and the cardio-vascular system. Dermal exposure is also of importance. However, healthy skin has a better barrier function when compared to the respiratory tract although this barrier function could be limited by skin lesions, strong mechanical strain or small nanoparticles (<5 to 10 nm). Exposure by ingestion is of lower concern within the workplace; following good personal hygiene and basic safety practice rules (such as washing hands with soap and water before breaks and at the end of the workday, not wearing personal protective clothing outside of work areas and not taking them home for cleaning) should avoid any oral uptake.
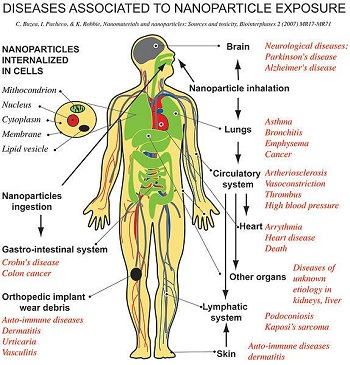
Figure 10: Diseases associated to nanoparticle exposure
Due to the size effects, smaller particles may diffuse faster in air than their larger counterparts and can get further down the respiratory tract. In addition, it is hard to remove nanoparticles from the body as they are known to cross mucus membranes. Many potential health effects (Fig. 10) include: · inflammation of airway (bronchitis, asthma, emphysema); · lung cancer; · neurodegenerative diseases (Parkinson's and Alzheimer's disease); · cardiovascular effects and heart disease; liver cancer; Crohn's disease and colon cancer.
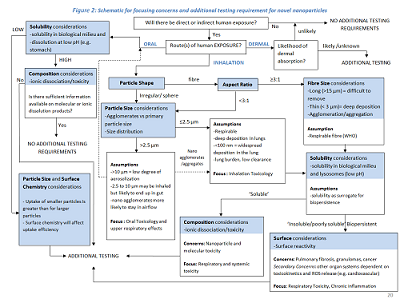
Although, presently, it is still uncertain which parameters represent the best predictive value for toxicity, there is growing evidence that for nanomaterials a high aspect ratio (HARNs) and poor water solubility might lead to negative effects on human health. In order to overcome the lack of specific data about the bio persistence of nanoparticles, water solubility is often used as a proxy for bio persistence. In terms of solubility only, poorly soluble or insoluble nanoparticles are considered of concern; soluble nanoparticles (with a water solubility above 100 mg/L) are considered of no concern. However, in some cases, a material may show a poor solubility in water but a good solubility in biological media as, for example, cobalt which is insoluble in water but soluble in serum. A categorization rating for the level of concern relating to the possible health hazard effects of nanoparticles based on the shape and persistence/water solubility characteristics is proposed in Table 4.
Concern Category High Medium - High Medium - Low Low
Table 4: Concern Categorisation Characteristics of nanoparticles
Poorly soluble or insoluble (water solubility <100 mg/L) Poorly soluble or insoluble (water solubility <100 mg/L) nanoparticles with specific toxicity and poorly soluble or insoluble HARNs Poorly soluble or insoluble nanoparticles with no specific toxicity Soluble nanoparticles--Examples
some carbon nanotubes nano-silver gold nanoparticles zinc oxide nanoparticles carbon black titanium dioxide sodium chloride nanoparticles lipid nanoparticles flour nanoparticles amorphous silica
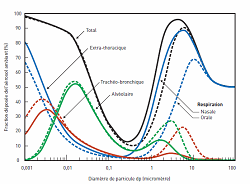
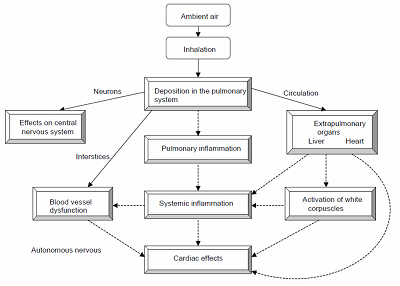
1) Inhalation Nanoparticles suspended in the air represent the greatest risk of exposure since they can breakdown to breathable-sized particles. Once nanoparticles are in the lungs, they can translocate to other [F25]organs or cells, providing an entryway to the body with the possibility of bio accumulation. The wall thickness on the capillaries and alveolar are approximately 0.5 µm thick, which allows airborne particles in the micro and nano-scale to be easily inhaled into the body. Exposure can be separated into three categories: more than 2.5 µm, less than 2.5 µm and less than 100 nm[F26]. (Fig. 11) Figure 11: Distribution of particles in airways (Picture obtained from - O. Witschger et al. INRS ND 2227, 2005) Micro-particles above 2.5 µm tend to settle in the upper airway and are released by mucociliary escalator or in the mucous lining of the bronchi and pushed out by the upper airway by coughing, sneezing, being blown out or swallowed.[F27] Micro-particles less than 2.5 µm penetrate deeper into the alveolar region and are only removed via an alveolar macrophage.[F28] Nano-particles less than 100 nm start to behave more like gas rather than a solid particulate. They begin to diffuse in the body by the pulmonary system. They have the ability to penetrate the lung and enter the bloodstream. When particles enter the blood stream, it allows them to be engulfed by cells and accumulate in organs like the heart, spleen, kidney, bone marrow, and liver[F29]. In addition some of these materials can also damage DNA and cause cancer (Fig. 12).Figure 12: Possible health effects of nanoparticles (Image from Oberdörster G., 2005)
Particular care is suggested when considering the risks that may be posed by nanoparticles possessing certain physical aspect characteristics. In particular, some HARNs demonstrated similarities in their physical characteristics to materials known to be hazardous, such as asbestos or some man-made mineral fibres. Studies suggested that HARNs may be retained in the pleural cavity for long periods of time if they show the additional characteristics: thinner than 3 m; longer than 10-20 m; bio persistent; do not dissolve/break into shorter fibres. As for nanofibres, concerns have been raised over nanoplatelets and their aerodynamic behaviour which might result in them penetrating deep within the lung. 2) Dermal Exposure The three layers of skin (epidermis, dermis and subcutaneous)[F30] make it difficult for ionic molecules to penetrate through. In addition, larger micro-particles cannot penetrate through the skin as well[F31]. However, nanoparticles can pass through the skin due to their smaller sizes (Fig. 13). They may pass through pores and go deep under the skin. Even if direct penetration of the skin has been reported for particles with a diameter of 1 m or less, most studies showed that nanoparticles can penetrate deeper skin layers only under unfavorable conditions (e.g. injured or stressed skin). Damaged skin (such as sun burns, eczema, acne and opened wounds) can accelerate the uptake of nanomaterials. Nanoparticles can also be absorbed to the bloodstream and bioaccumulate into organs. They can be very toxic to tissues by increasing oxidative stress and inflammation of cytokine production resulting to cell death.
Figure 13: Penetration of gold nanoparticles through intact human skin (Image from F.L. Filon et al., Nanotox. Online, 2011, pp.1-9)
3) Ingestion Nanoparticles can be inadvertently consumed orally and passes through the gastrointestinal track. An increase of absorption in the bloodstream is observed due to the increased surface area compared to the skin. In addition, smaller nanoparticles are easily absorbed compared to their larger counterpart. In general, only the smaller nanoparticles are absorbed this way but the extent of absorption levels are unknown. Ingestion usually occurs via a hand-to-mouth transfer of unwashed materials/hands or contaminated food and drinks[F32]. The adverse effects of ingested nanoparticles are still no completely understood. However, the materials have been observed to accumulate in the liver, spleen, kidney and may cause permanent liver damage.[F33]
Both carbon-containing and metal dusts can explode if they are aerosolized at a high enough concentration and if oxygen and an ignition source are present. Accidental explosions have been reported involving metal nanopowders that have resulted in deaths of workers. Because the surface-to-volume ratio increases as a particle becomes smaller, nanoparticles may be more prone to explosion than an equivalent mass concentration of larger particles.[F34] Dust explosions can occur where any powdered combustible material is present in an enclosed atmosphere or, in general, in high enough concentrations of dispersed combustible particles in atmosphere or other suitable gaseous medium such as molecular oxygen. Bench-scale research should present a low risk of explosion compared to work in pilot plants or full-scale manufacturing facilities. Nonetheless, researchers should avoid creating concentrated aerosols of combustible nanoparticles. Decreasing the particle size of combustible materials can increase combustion potential and combustion rate, leading to the possibility of relatively inert materials becoming highly reactive in the nanometer size range. Some nanoparticles are designed to generate heat through the progression of reactions at the nanoscale. Such materials may present a fire hazard that is unique to engineered nanoparticles. One example is aluminum powder; particles bigger than 80 nm are known to be inert when exposed to air / oxygen. On the opposite, particles less than 80 nm are known to be very reactive and can cause explosion. Another example is nanoscale Al/MoO3 particles that can ignite more than 300 times faster than corresponding micrometer-scale material.
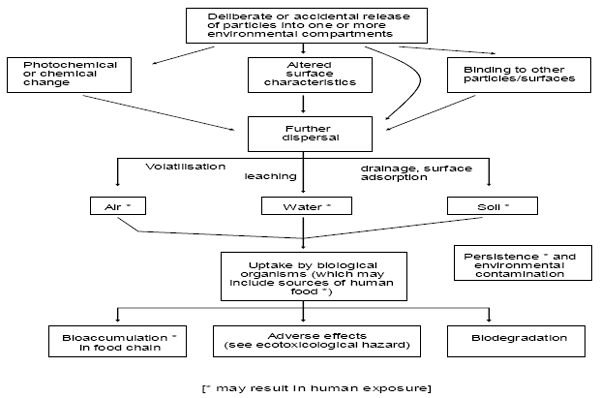
There are limited publications on the effects of engineered nanoparticles on animals and plants in the environment. To date only few quantitative analytical techniques for measuring nanoparticles in natural systems are available, which results in a serious lack of information about their occurrence in the environment. The main concern will be if any of the nanoparticles entering the environment are toxic or could become toxic to living species in the environment. For example, there is the possibility of nanoparticles being toxic to microorganisms in the soil and groundwater. Following on from this would be possible hazards from the nanoparticles or from consuming the microorganisms affected by the nanoparticles for fish, insects or mammals. There is also a risk to plants from nanoparticles which again could have a follow-on effect on the food chain. For example the deposition of atmospheric particles on crops could provide another route for toxic or reactive nanoparticles into the food chain [F36](Fig. 14). Release of nanoparticles may come from point sources such as production facilities, landfills, or wastewater treatment plants or from nonpoint sources such as wear from materials containing NP[F37]. Accidental release during production or transport is also possible. In addition to the unintentional release there are also nanoparticles released intentionally into the environment. A number of studies have examined the uptake and effects of nanoparticles at a cellular level to evaluate their impact on humans; it can reasonably be assumed that the conclusions of these studies may be extrapolated to other species, but more research is needed to confirm this assumption. As a fact, a consistent body of evidence shows that nanosized particles are taken up by a wide variety of mammalian cell types and are able to cross the cell membrane and become internalized[F38]. Results from eco-toxicological studies show that certain nanoparticles have effects on organisms under environmental conditions, though mostly at elevated concentrations. However, persistent insoluble nanoparticles may cause problems in the environment that are much greater than those revealed by human health assessments.
Figure 14: Fate of Nanoparticles in the environment (Image from: "The appropriateness of existing methodologies to assess the potential risks associated with engineered and adventitious products of nanotechnologies", European Commission Health & Consumer Protection Directorate-General, March 2006)
5) Risk Assessment and Safety Precautions for Nanomaterial Uses
The risk of toxicity can be determined using this formula: = Exposure is defined as the chance of release for a specific material. Since engineered nanoparticles are relatively new, they are to be considered toxic and the potential of an exposure must be investigated to minimize the risk. Routine workplace activities and other foreseeable events (e.g. accidental spills or other equipment failure scenarios) which may potentially result in the release of nanoparticles and subsequent exposure of workers should be defined. A list of lab activities which can present a potential risk of exposure could consist of: material reception, unpacking, delivery or shipment; laboratory operations(e.g. weighing); cleaning and maintenance; storage and packaging; waste management; possible emergency situations (e.g. spill).
a) Risk Assessment and SOPs
Risk assessment is defined as the systematic process of evaluating the potential risk of an activity or a project. It is important to take a good health/safety approach when working with engineered nanoparticles. This can be done by following these 4 elemental steps: i. ii. iii. iv. Identifying the hazard Assessing the risk Preventing or controlling the risk Evaluating the effectiveness of the control measures
Risk assessment should be conducted based on the type of nanoparticles being used (e.g. a suspended solution vs. a dry powder). When conducting an assessment, one should always refer to the MSDS/SDS (Material Safety Data Sheet or Safety Data Sheet) of the material being used. Currently, specific information on the physicochemical characteristics of nanoparticles is not always reported in MSDS/SDS. Moreover, toxicological and ecotoxicological data specific to nanoparticles may be lacking. However, if the macro form of a substance is classified as a carcinogen, mutagen or reproductive toxin, as a sensitizer or for another significant toxicity, then it should be assumed that the nanoform will also show these properties, unless proven otherwise. Risk assessment tools, such as control banding are available from different organism web sites. Control banding is a qualitative strategy for assessing and managing hazards associated with chemical exposures in the workplace (Fig. 15). The concept is used to manage exposures to potentially hazardous materials through the application recommended control approaches. Such tools may help in assessing the level of risks associated with nanoparticles work. Laboratories intending to commence projects involving nanoparticles are asked to contact EHS and request a hazard assessment for their location. EHS will require that labs preparing to work with nanoparticles prepare and submit SOPs for approval before beginning to handle the material.-Figure 15: Example of control banding tool (obtained from The GoodNanoGuide www.goodnanoguide.org)
As for any potentially hazardous materials, there are several methods to use in controlling a nanoparticles exposure. 1) Elimination or substitution This method consists of eliminating or substituting the toxic components being used. However, this method is not always feasible since the material of study may be toxic. This approach can be tackled by changing some aspects of process, such as: 2) using a liquid suspension instead of a free powder using of less toxic solvents Engineering controls--This method consists of using an exhaust or containment system to protect oneself from an exposure risk. Using this method strongly depends on the system that is being utilized (Fig. 16). When working with an exhaust system, a HEPA (High Efficiency Particulate Air filter; 99.97% efficiency for 300 nm particles) or ULPA filter (Ultra Low Penetration Air filter; 99.999% efficiency of 120 nm particles) must be used. Note that the general exhaust systems used at Concordia University do not have HEPA or ULPA filters.
Examples of exhaust systems equipped with HEPA or ULPA filtering devices that can be used with nanoparticles (Fig. 17) are: Nano-safety cabinet (Labconco); Biological safety cabinet; Table-top HEPA filtration unit.--An alternative engineering control is to use a glove box or an enclosed system (Fig. 18). The primary advantage of using a glovebox is the protection it affords; when used properly to manipulate nanoparticle powders. The disadvantages of using a glovebox relate to the extra time required to move materials and equipment in and out of the enclosure, the difficulty of manipulating nanomaterials when wearing gloves, and the need to periodically clean the enclosure. The two most likely sources of exposure when using a glovebox are the transfer of materials into and out of the box and the cleaning of the box following its use. If a positive pressure using an inert atmosphere (e.g. nitrogen or argon) needs to be maintained in the enclosed system, one has to make sure that any exhaust getting out of the enclosure must be connected to a HEPA or ULPA unit in order to avoid any potential leak of nanoparticles.
3) Safe laboratory work practices Those practices include: awareness or safety training for students, staff, employees or anyone involved working with nanoparticles; development and application of standard operating procedures (SOPs) when working with specific nanoparticles; work area restriction for non-users or unauthorized personnel; no eating or drinking in the work laboratory; good sanitary practices, such as frequent hand washing; clear work-zone delimitation (e.g. signs or marking tape) within the laboratory area specific for nanoparticles/nanomaterial work: high-circulating areas, such as areas close to doors or hallways should be avoided. In addition, clean room mats or tacky mats can be used to reduce the spreading of nanoparticles. These mats help trap impurities for locations that require stringent dust and dirt control. The tacky surface removes particles from shoes avoiding therefore spreading of nanoparticles in nanoparticles-free areas. Other tolls such as anti-static devices can also be used when handling dry powders (Fig. 19) to avoid spreading of air born particles.
4) Personal Protective Equipment (PPE) Even though Personal protective equipment (PPE) should never be considered as the first line of defense against contaminants, nevertheless they must always be used in order to minimise users' exposure to nanoparticles. Basic nanoparticles PPE should include: Hand protection Gloves must always be worn when handling dry nanoparticles or nanoparticles suspension in various solvents. Even though studies have demonstrated that most types of gloves (latex, nitrile, vinyl, etc.) offer a good barrier against most nanoparticles in the powder form, nitrile gloves proved to offer an increased protection when suspension in solvents are used. Double gloving is highly recommended for extensive use and gloves with gauntlets (extended sleeves) reduce risks of skin exposure and therefore offer a better protection (Fig. 20). Disposable gloves should be changed routinely or at the first sign of contamination. Eye protection Safety glasses are mandatory in all Concordia University laboratories and should therefore be the minimal eye protection to be worn when appropriate engineering controls (e.g. nano-cabinet or glove box) is being used. However, safety goggles offer an increased eye protection compared to safety glasses and should be favored when: appropriate engineering controls are deficient or not available; o a heavy use or large amounts of nanoparticles are being used; a suspension of nanoparticles in solvents is being generated and splash or aerosol generation is possible.
Protective clothing It was demonstrated the nanoparticles could get through most of regular woven fabrics (e.g. cotton).[F39] Therefore, regular lab coats do not offer an appropriate protection from nanoparticles. Impervious disposable clothing, such as Tyvek® lab coats, sleeves and shoe covers should rather be used (Fig. 21). Their fabrics are shown to be impermeable to nanoparticles. If a head scarf is worn by the lab user, a Tyvek® hood should be worn on top of it to avoid possible contamination of the cloth.
Protecting clothing should be stored and donned in a dedicated "buffer" area in lab area in order to avoid cross-contamination. Protection clothing should not be worn outside lab and non-disposable work clothes should not be taken home for cleaning; re-usable protective clothing should rather be laundered through department laundering services. Disposable protecting clothing (gloves and Tyvek® gear) should be disposed of as chemical waste. · Respiratory protection Respiratory protection should be worn when working with dry nano-powders and when appropriate engineering controls (e.g. nano-safety cabinet) are deficient or not available. Here are descriptions of different types of respirators or masks available on the market along with their intended use; it has to be noted that these respirators cannot be used in areas of low oxygen concentration (i.e. an oxygen deficient atmosphere). Surgical masks Those are loose-fitting and disposable masks preventing the release of contaminants from the user to the environment. They are not meant to protect against dust or nanoparticles and should therefore not be used in laboratories using nanoparticles. o Filtering Face-piece Respirators (FFRs) Those are the most commonly used disposable respiratory masks and they protect user from dusts and aerosols according to their NIOSH certification: N : not resistant to oil particles R : somewhat resistant to oil particles (8 hours of continuous or intermittent use) P : strongly resistant to oil particles(oil proof) 95, 99 or 100 : protection rating (%) in NIOSH test FFRs do not act as sieves but rather intercept nanoparticles using different types of filtering mechanisms; impaction, interception, diffusion and electrostatic attraction (when electret filter media is used). Once the particles are trapped within the FFRs filter media, they cannot be removed (not a reversible process). Several tests performed by different institutes (e.g. NIOSH, OSHA, IRSST...) demonstrated that typical N95 masks met the NIOSH filtering standards (< 5% penetration for particles less than 100 nm) offering therefore an adequate protection against nanoparticles. However, their efficacy was greatly affected by humidity and breathing rate. The main safety issue observed with the disposable FFRs resides in achieving proper seal around nose and mouth. Improper use or bad work practices by workers (e.g. putting de FFRs on top of the head) often results in a lose fit which allow nanoparticles to enter the mask (Fig. 22). Therefore, their use for work with nanoparticles is not recommended by EHS. o Half or full-face respirators Half or full-face respirators consist of a rubber (i.e. silicone) mask, worn over the nose and mouth and held in place by adjustable straps which go over and behind the user's head (Fig. 23). The respirators are fitted with two removable filter cartridges. These units are heavier than the disposable FFRs, but provide far better protection for the wearer, provided that they are fitted properly. Anyone requiring to wear a half or fullface respirator must previously get fit-tested by EHS. This can be done contacting EHS at ehs@concordia.ca.
The respirators require periodic maintenance and cleaning. They are washable and reusable but should not be shared among users. They require filter cartridges adapted to risk.-When working with nanoparticles in the absence of appropriate engineering controls, EHS recommends the use of a half-face respirator since it offers a better seal then disposable masks. The half-face respirator should be equipped with P100 filter cartridges which are comparable to HEPA filters. Studies demonstrate that they are good in protecting users from nanoparticles with sizes down to 2.5 nm. 6) Storage, Waste Handling, Waste Issues and Spills
a) Storage
Nanoparticles, nanomaterials and nanopowders must be stored in closed and sealed containers at all times, in a cool and well-ventilated area clearly identified (e.g. `Nanomaterials storage area `POTENTIALLY TOXIC'). If possible, a lab cabinet restricted to nanomaterials storage only should be dedicated. All containers with nanoparticles in should be clearly identified (e.g. Nanoscale Gold Nanoparticles). If the nanoparticles are received in plastic (Ziploc) bags from the supplier, the bag should be placed in a secondary hard sealable container. Nanoparticles and nanopowders should be stored away from acids, oxidizers and other metals.
b) Waste Handling
There are currently no specific regulation or guidelines for proper disposal of nanoparticles. It is therefore important to handle nanoparticles very cautiously since they are to be considered as toxic. Any waste containing nanoparticles or nanomaterials should never be disposed of in regular garbage and flushed down the drain. Nanoparticles should be disposed of following EHS chemical waste guidelines: any contaminated objects (paper, wipes, tips) should be disposed of as solid chemical waste; any contaminated disposable PPE (e.g. gloves, disposable lab coats) should be disposed of as solid chemical waste; pure nanomaterials/nanoparticles in the solid (including powder) form should also be disposed of as chemical waste; nanoparticles in solution can be disposed of as liquid chemical waste. All waste containers should be properly labelled, mentioning that they contain nanoparticles and should be properly sealed once full and ready to be picked up. For any concerns about nanomaterials waste, please contact EHS at hazardouswaste@concordia.ca.
c) Spills
The potential for inhalation exposure during cleanup will be influenced by the likelihood of nanoparticles becoming airborne, with powder form presenting a greater inhalation potential than the solution forms. Small nanoparticle spills that occur on non-porous surfaces (e.g. linoleum, stainless steel, hardwood flooring) can be cleaned by trained lab personnel. However, if the spill occurs on a porous surface or item (e.g. rug or mat), it might become difficult to fully decontaminate this surface and therefore the item should be discarded along with other nanomaterial waste. 1) In the event of a small spill If lab users are within their comfort zone or have been trained for spill response, they can clean up the spill using the following techniques: Any solid nanoparticles should be cleaned up using wet wiping.-Dry sweeping or air hoses should not be used to clean work areas. If dry vacuuming is to be used, only a vacuum cleaner equipped with a HEPA is to be used. Absorbent wipes for liquid suspensions.
NIOSH recommends the following equipment included in a nanomaterial spill kit to be readily available in or near each laboratory working with such materials. A nanomaterial spill kit for a laboratory environment may contain the following: Barricade tape Nitrile gloves Elastomeric respirator with appropriate filters Adsorbent material Wipes Sealable plastic bags Walk-off mat (e.g., Tacki-Mat®) Spray bottle with deionized water or other appropriate liquid Procedure: 1. Clean-up the spill immediately after it has occurred. 2. Prevent the spread of the spilled nanomaterials. DO NOT allow people to walk through spill area. 3. Wear PPE (like disposable gloves and shoe covers or place double plastic bags over your shoes) during the clean up. 4. Place the collected nanoparticles into a clearly labelled and sealed chemical waste container. 5. Contact EHS (hazardouswaste@concordia.ca) for waste pick-up. 2) In the event of a large spill Large spills or spills of highly toxic or dangerously reactive materials should not be handled by lab users. In the event of any large spills, lab users should readily contact Security at extension 3717. Procedure: 1. Advise and warn co-worker 2. Help contaminated or injured persons. 3. Evacuate the spill area and do not touch the hazardous material 4. Keep others from entering contaminated area. 5. Notify Security at extension 3717 or 514-848-3717 and provide them with the following information: Location of spill; Name of hazardous material; Quantity involved; Related health hazards and precautions to be taken.
6. Provide Material Safety Data Sheet or Safety Data Sheet (MSDS/SDS) or appropriate documentation.
Lab users need to remain available to the Emergency Response Team or Security in order to provide assistance. An Injury/near-miss report must be filled up in the event of any types of spill. Injury/near miss reports can be found on the EHS website in the injury report section.
7) Emergency Procedures
Nanoparticles and nanomaterials toxicity are often found to be chemical-specific. The effects of exposure to nanoparticles may therefore vary depending on the type of materials being used (e.g. nanoclays, carbon nanotubes or metals). One must therefore always refer to the MSDS/SDS of the chemical being used. Here are some general guidelines in the event of exposure.
a) Skin Contact
1. 2. 3. 4. 5. Remove any contaminated clothing immediately. Wash affected area with soap or mild detergent and large amounts of water until no evidence of the nanomaterial remains (15-20min). Refer to MSDS/SDS of material. Contact Security ext. 3717 to get medical attention. Any contaminated clothing should be washed before reuse.
b) Eye Contact
1. 2. 3. Immediately rinse eyes and inner surface of eyelid with water for 15 minutes by forcibly holding the eye open. Refer to MSDS/SDS of material. Contact Security ext. 3717 to get medical attention.
c) Inhalation
1. 2. 3. 4. 5. Remove from exposure and move affected person to fresh air immediately. Refer to MSDS/SDS of material. Contact Security ext. 3717 to get medical attention. If breathing is difficult, give oxygen. If breathing has ceased, apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask; never give direct mouth-to-mouth resuscitation to avoid any potential contamination.
d) Ingestion
1. 2. 3. Rinse inside of mouth with water. Do not induce vomiting. Refer to MSDS/SDS of material.
28 / 30
4. 5.
Never give anything by mouth to an unconscious person. Contact Security ext. 3717 to get medical attention.
All nanomaterial incidents must be reported to your Supervisor and to EHS. An Injury/near-miss report must be filled up for any incident involving nanomaterials/nanoparticles spill or exposure. Injury/near miss reports can be found on the EHS website in the injury report section. If you have any concerns about the use of nanoparticles or nanomaterials at Concordia University, please contact EHS at ehs@concordia.ca
References: National Institute of Environmental Health and Safety http://www.niehs.nih.gov/health/topics/agents/sya-nano/ CAUT Health and SafetyFact Sheet http://www.caut.ca/docs/default-source/health-safety-fact-sheets/animal-hazards.pdf?sfvrsn=8 Florida State University Laboratory Safety http://www.safety.fsu.edu/lab-nano.html University of Dayton Environmental Health and Safety/Risk Management Nanotechnology Safety http://campus.udayton.edu/~UDCampusPlanning/EHSRM/index.php?option=com_content&view=art icle&id=18&Itemid=205 National Institutes of Health Office of Research Services Division of Occupational Health and Safety http://www.ors.od.nih.gov/sr/dohs/Documents/Nanotechnology%20Safety%20and%20Health%20Pr ogram.pdf National Collaborating Center for Environmental Health - Nanotechnology: A Review of Exposure, Health Risks and Recent Regulatory Developments http://www.ncceh.ca/sites/default/files/Nanotechnology_Review_Aug_2011.pdf Scientific Committee on Emerging and Newly Identified Health Risks - Risk Assessment of Products of Nanotechnologies http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_023.pdf European Commission - Guidance on the protection of the health and safety of workers from the potential risks related to nanomaterials at work https://osha.europa.eu/en/news/eu-safe-use-of-nanomaterials-commission-publishes-guidance-foremployers-and-workers Safe Work Australia Safety Hazards of Engineered Nanomaterials Information Sheet http://www.safeworkaustralia.gov.au/sites/SWA/about/Publications/Documents/762/Safetyhazards-engineered-nanomaterials.pdf IRSST Nanomatériaux; Guide de bonnes pratiques favorisant la gestion des risques en milieu de travail, 2e edition, R-840 http://www.irsst.qc.ca/-publication-irsst-nanomateriaux-guide-r-840.html Books: Adverse Effects of Engineered Nanomaterials: Exposure, Toxicology, and Impact on Human Health
Quantum Dots
Quantum dots are tiny particles or nanocrystals of a semiconducting material with diameters in the range of 2-10 nanometers (10-50 atoms). They were first discovered in 1980.1 Quantum dots display unique electronic properties, intermediate between those of bulk semiconductors and discrete molecules, that are partly the result of the unusually high surface-to-volume ratios for these particles.2-4 The most apparent result of this is fluorescence, wherein the nanocrystals can produce distinctive colors determined by the size of the particles.
Due to their small size, the electrons in quantum dots are confined in a small space (quantum box), and when the radii of the semiconductor nanocrystal is smaller than the exciton Bohr radius (exciton Bohr radius is the average distance between the electron in the conduction band and the hole it leaves behind in the valence band), there is quantization of the energy levels according to Pauli’s exclusion principle (Figure 1).5,6 The discrete, quantized energy levels of quantum dots relate them more closely to atoms than bulk materials and have resulted in quantum dots being nicknamed 'artificial atoms'. Generally, as the size of the crystal decreases, the difference in energy between the highest valence band and the lowest conduction band increases. More energy is then needed to excite the dot, and concurrently, more energy is released when the crystal returns to its ground state, resulting in a color shift from red to blue in the emitted light. As a result of this phenomenon, quantum dots can emit any color of light from the same material simply by changing the dot size. Additionally, because of the high level of control possible over the size of the nanocrystals produced, quantum dots can be tuned during manufacturing to emit any color of light.7
Quantum dots can be classified into different types based on their composition and structure.
Figure 1. Splitting of energy levels in quantum dots due to the quantum confinement effect, semiconductor band gap increases with decrease in size of the nanocrystal.
Core-Type Quantum DotsQuantum dots can be single component materials with uniform internal compositions, such as chalcogenides (selenides or sulfides) of metals like cadmium or zinc, example, CdSe (Aldrich Prod. No. 662550) or CdS (Aldrich Prod. No. 662593). The photo- and electroluminescence properties of core-type nanocrystals can be fine-tuned by simply changing the crystallite size.
Core-Shell Quantum DotsThe luminescent properties of quantum dots arise from recombination of electron-hole pairs (exciton decay) through radiative pathways. However, the exciton decay can also occur through nonradiative methods, reducing the fluorescence quantum yield. One of the methods used to improve efficiency and brightness of semiconductor nanocrystals is growing shells of another higher band gap semiconducting material around them. These quantum dots with small regions of one material embedded in another with a wider band gap are known as core-shell quantum dots (CSQDs) or core-shell semiconducting nanocrystals (CSSNCs). For example, quantum dots with CdSe in the core and ZnS in the shell (Aldrich Product Nos. 731838, 731870) available from Aldrich Materials Science exhibit greater than 80% quantum yield. Coating quantum dots with shells improves quantum yield by passivizing nonradiative recombination sites and also makes them more robust to processing conditions for various applications. This method has been widely explored as a way to adjust the photophysical properties of quantum dots.8-10
Alloyed Quantum DotsThe ability to tune optical and electronic properties by changing the crystallite size has become a hallmark of quantum dots. However, tuning the properties by changing the crystallite size could cause problems in many applications with size restrictions. Multicomponent quantum dots offer an alternative method to tune properties without changing crystallite size. Alloyed semiconductor quantum dots with both homogeneous and gradient internal structures allow tuning of the optical and electronic properties by merely changing the composition and internal structure without changing the crystallite size. For example, alloyed quantum dots of the compositions CdSxSe1-x/ZnS of 6nm diameter emits light of different wavelengths by just changing the composition (Aldrich Prod. Nos. 753742, 753793) (Figure 2). Alloyed semiconductor quantum dots formed by alloying together two semiconductors with different band gap energies exhibited interesting properties distinct not only from the properties of their bulk counterparts but also from those of their parent semiconductors. Thus, alloyed nanocrystals possess novel and additional composition-tunable properties aside from the properties that emerge due to quantum confinement effects.11
Figure 2. Photoluminescence of alloyed CdSxSe1-x/ZnS quantum dots of 6nm diameter. The material emits different color of light by tuning the composition.
The unique size and composition tunable electronic property of these very small, semiconducting quantum dots make them very appealing for a variety of applications and new technologies.12
Quantum dots are particularly significant for optical applications owing to their bright, pure colors along with their ability to emit rainbow of colors coupled with their high efficiencies, longer lifetimes and high extinction coefficient. Examples include LEDs and solid state lighting, displays and photovoltaics.7,13,14
Being zero dimensional, quantum dots have a sharper density of states than higher-dimensional structures. Their small size also means that electrons do not have to travel as far as with larger particles, thus electronic devices can operate faster. Examples of applications taking advantage of these unique electronic properties include transistors, solar cells, ultrafast all-optical switches and logic gates, and quantum computing, among many others.13-15
The small size of quantum dots allow them to go anywhere in the body making them suitable for different bio-medical applications like medical imaging, biosensors, etc. At present, fluorescence based biosensors depend on organic dyes with a broad spectral width, which limits their effectiveness to a small number of colors and shorter lifetimes to tag the agents. On the other hand, quantum dots can emit the whole spectrum, are brighter and have little degradation over time thus proving them superior to traditional organic dyes used in biomedical applications.16
Aldrich Materials Science offers semiconductor, core-shell and alloyed quantum dots tuned to emit different colors in the visible spectrum while exhibiting high quantum yield. Nanocrystals are available in both aqueous and organic formulations suitable for use in different applications.
Find the complete list of Quantum Dots offered by Aldrich Materials Science here.
References1. Ekimov, A. I.; Onushchenko, A. A. JETP Lett. 1981, 34, 345–349.
2. Kastner, M. A. Physics Today, 1993, 46(1), 24.
3. Ashoori, R. C. Nature, 1996, 379(6564), 413.
4. Collier, C. P.; Vossmeyer, T.; Heath, J. R. Annual Review of Physical Chemistry, 1998, 49, 371.
5. Reimann, S. M.; Manninen, M. Reviews of Modern Physics, 2002, 74(4), 1283.
6. Bawendi, M. C.; Steigerwald, M. L.; Brus, L. E. Annual Review of Physical Chemistry, 1990, 41, 477.
7. Yoffe, A. D. Advances in Physics, 2001, 50(1), 1.
8. Bailey, R. E.; Nie, S. Edited by Rao, C. N. R.; Mueller, A.; Cheetham, A. K. Chemistry of Nanomaterials, 2004, 2, 405.
9. Dorfs, D.; Eychmueller, A. Zeitschrift fuer Physikalische Chemie, 2006, 220(12), 1539.
10. Smith, A. M.; Nie, S. Nature Biotechnology, 2009, 27(8), 732.
11. Vastola, G.; Zhang, Y.-W.; Shenoy, V. B. Current Opinion in Solid State & Materials Science, 2012, 16(2), 64.
12. Vahala, K. J. Nature, 2003, 424(6950), 839.
13. Nirmal, M.; Brus, L. Accounts of Chemical Research, 1999, 32(5), 407.
14. Sargent, E. H. Nature Photonics, 2012, 6(3), 133.
15. Zhao, Y.; Burda, C. Energy & Environmental Science, 2012, 5(2), 5564.
16. Medintz, I. L.; Uyeda, H. T.; Goldman, E. R.; Mattoussi, H. Nature Materials, 2005, 4(6), 435.
- See more at: http://www.sigmaaldrich.com/materials-science/nanomaterials/quantum-dots.html#sthash.rZO3jKbJ.dpuf
[F2]Dendrimers are highly branched, star-shaped macromolecules with nanometer-scale dimensions. Dendrimers are defined by three components: a central core, an interior dendritic structure (the branches), and an exterior surface with functional surface groups. The varied combination of these components yields products of different shapes and sizes with shielded interior cores that are ideal candidates for applications in both biological and materials sciences. While the attached surface groups affect the solubility and chelation ability, the varied cores impart unique properties to the cavity size, absorption capacity, and capture-release characteristics. Applications highlighted in recent literature include drug delivery, gene transfection, catalysis, energy harvesting, photo activity, molecular weight and size determination, rheology modification, and nanoscale science and technology.1, 2 Monodisperse dendrimers are synthesized by step-wise chemical methods to give distinct generations (G0, G1, G2, ...) of molecules with narrow molecular weight distribution, uniform size and shape, and multiple (multivalent) surface groups Z. Dendrons are monodisperse wedge-shaped sections of dedrimers with a single focal point reactive function. Hyperbranched polymers are polydisperse dendritic macromolecules synthesized by lower-cost polymerization methods.
(1) Functional Polymers and Dendrimers: From Synthesis to Application.
Proceedings of the American Chemical Society Division of Polymeric Materials: Science & Engineering, San Diego, CA, April 1-5, 2001; ACS, 2001
(2) Dendrimers and Other Dendritic Polymers, Jean M.J. Frechet and Donald A. Tomalia, J. Wiley & Sons (2001)
- See more at: http://www.sigmaaldrich.com/materials-science/material-science-products.html?TablePage=16375655#sthash.Vk362d8T.dpuf
[F3]They are molecules composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube.Spherical fullerenes are sometimes called buckyballs, the C60 variant is often compared to a typical white and black soccer football.
Cylindrical fullerenes are called buckytubes.
Recently discovered is the "buckyegg", by researchers at UC Davis.
Fullerenes are similar in structure to graphite, which is composed of a sheet of linked hexagonal rings, but they contain pentagonal (or sometimes heptagonal) rings that prevent the sheet from being planar.
[F4]Nano Interacting with proteins ( gene’s rna –dna-chromosomes-muscle tissue-organs-etc)
[F5]This is being referred to for medical but the same thing can be utilized in a military fashion or to even make it look like a pandemic or outbreak
[F6]So in this case size does matter and the smaller the size the more lethal
[F7]Different particles will impact health all differently but negatively
[F8]Construct of nano is as well a determining factor to toxiciity
[F9]Granular material such as sand, gravel, crushed gravel, crushed stone, slag, and cinders. Aggregate is used in construction for the manufacturingof concrete, mortar, grout, asphaltic concrete, and roofing shingles. It is also used in leaching fields, drainage systems, roof ballast, landscaping, and as a base course for pavement and grade slabs. Aggregate is classified by size and gradation.
[F10]The Term “Migrate” is an interesting choice of words meaning movement and indicating further that these things has some means of propulsion
[F11]So now the question is how long is the process of adaption and does this mean actually co existing or does it mean it will eventually over time break it down
[F12]This would indicate that if the sprays are using glyphosate s with nano then the GMO ‘s are essentially killing people via the agribacteurim to the plant itself and through the root structure and plant crop
[F13]Glyphosates use nano adujants to be sprayed on crops there is nano silver being used as a pesticide in the field and iin food production as well~ and then there is the chemtrails which are using carbon nano fibres as well as nano silver and other nano metals which eventually through rain or wind or gravity eventually make there way into the eco system
[F14]Buckminsterfullerene (or bucky-ball) is a spherical fullerene molecule with the formula C60. It has a cage-like fused-ring structure (truncated icosahedron) which resembles a football (soccer ball), made of twenty hexagons and twelve pentagons, with a carbon atom at each vertex of each polygon and a bond along each polygon edge.It was first generated in 1985 by Harold Kroto, James R. Heath, Sean O'Brien, Robert Curl, and Richard Smalley at Rice University.[2] Kroto, Curl and Smalley were awarded the 1996 Nobel Prize in Chemistry for their roles in the discovery of buckminsterfullerene and the related class of molecules, the fullerenes. The name is a reference to Buckminster Fuller, as C60 resembles his trademark geodesic domes. Buckminsterfullerene is the most common naturally occurring fullerene molecule, as it can be found in small quantities in soot.[3][4] Solid and gaseous forms of the molecule have been detected in deep space.[5] Buckminsterfullerene is one of the largest objects to have been shown to exhibit wave–particle duality; as stated in the theory every object exhibits this behavior.[6][7] Its discovery led to the exploration of a new field of chemistry, involving the study of fullerenes.
[F15]The speculation is valid and warrants a huge investigation based on what has been said in regard to mobility of the fullerenes it is not a stretch to conclude that the food supply Is laced with this and that the toxins it to would bind with and morph with would also then be introduced into the food and water tables
[F16]They are molecules composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube.Spherical fullerenes are sometimes called buckyballs, the C60 variant is often compared to a typical white and black soccer football.
Cylindrical fullerenes are called buckytubes.
Recently discovered is the "buckyegg", by researchers at UC Davis.
Fullerenes are similar in structure to graphite, which is composed of a sheet of linked hexagonal rings, but they contain pentagonal (or sometimes heptagonal) rings that prevent the sheet from being planar.
[F17]Quantum dots are tiny particles or nanocrystals of a semiconducting material with diameters in the range of 2-10 nanometers (10-50 atoms). They were first discovered in 1980.1 Quantum dots display unique electronic properties, intermediate between those of bulk semiconductors and discrete molecules, that are partly the result of the unusually high surface-to-volume ratios for these particles.2-4 The most apparent result of this is fluorescence, wherein the nanocrystals can produce distinctive colors determined by the size of the particles.
Due to their small size, the electrons in quantum dots are confined in a small space (quantum box), and when the radii of the semiconductor nanocrystal is smaller than the exciton Bohr radius (exciton Bohr radius is the average distance between the electron in the conduction band and the hole it leaves behind in the valence band), there is quantization of the energy levels according to Pauli’s exclusion principle (Figure 1).5,6 The discrete, quantized energy levels of quantum dots relate them more closely to atoms than bulk materials and have resulted in quantum dots being nicknamed 'artificial atoms'. Generally, as the size of the crystal decreases, the difference in energy between the highest valence band and the lowest conduction band increases. More energy is then needed to excite the dot, and concurrently, more energy is released when the crystal returns to its ground state, resulting in a color shift from red to blue in the emitted light. As a result of this phenomenon, quantum dots can emit any color of light from the same material simply by changing the dot size. Additionally, because of the high level of control possible over the size of the nanocrystals produced, quantum dots can be tuned during manufacturing to emit any color of light.7
Quantum dots can be classified into different types based on their composition and structure.
- See more at: http://www.sigmaaldrich.com/materials-science/nanomaterials/quantum-dots.html#sthash.rZO3jKbJ.dpuf
Quantum Dots
Quantum dots are tiny particles or nanocrystals of a semiconducting material with diameters in the range of 2-10 nanometers (10-50 atoms). They were first discovered in 1980.1 Quantum dots display unique electronic properties, intermediate between those of bulk semiconductors and discrete molecules, that are partly the result of the unusually high surface-to-volume ratios for these particles.2-4 The most apparent result of this is fluorescence, wherein the nanocrystals can produce distinctive colors determined by the size of the particles.
Due to their small size, the electrons in quantum dots are confined in a small space (quantum box), and when the radii of the semiconductor nanocrystal is smaller than the exciton Bohr radius (exciton Bohr radius is the average distance between the electron in the conduction band and the hole it leaves behind in the valence band), there is quantization of the energy levels according to Pauli’s exclusion principle (Figure 1).5,6 The discrete, quantized energy levels of quantum dots relate them more closely to atoms than bulk materials and have resulted in quantum dots being nicknamed 'artificial atoms'. Generally, as the size of the crystal decreases, the difference in energy between the highest valence band and the lowest conduction band increases. More energy is then needed to excite the dot, and concurrently, more energy is released when the crystal returns to its ground state, resulting in a color shift from red to blue in the emitted light. As a result of this phenomenon, quantum dots can emit any color of light from the same material simply by changing the dot size. Additionally, because of the high level of control possible over the size of the nanocrystals produced, quantum dots can be tuned during manufacturing to emit any color of light.7
Quantum dots can be classified into different types based on their composition and structure.
Figure 1. Splitting of energy levels in quantum dots due to the quantum confinement effect, semiconductor band gap increases with decrease in size of the nanocrystal.
Core-Type Quantum DotsQuantum dots can be single component materials with uniform internal compositions, such as chalcogenides (selenides or sulfides) of metals like cadmium or zinc, example, CdSe (Aldrich Prod. No. 662550) or CdS (Aldrich Prod. No. 662593). The photo- and electroluminescence properties of core-type nanocrystals can be fine-tuned by simply changing the crystallite size.
Core-Shell Quantum DotsThe luminescent properties of quantum dots arise from recombination of electron-hole pairs (exciton decay) through radiative pathways. However, the exciton decay can also occur through nonradiative methods, reducing the fluorescence quantum yield. One of the methods used to improve efficiency and brightness of semiconductor nanocrystals is growing shells of another higher band gap semiconducting material around them. These quantum dots with small regions of one material embedded in another with a wider band gap are known as core-shell quantum dots (CSQDs) or core-shell semiconducting nanocrystals (CSSNCs). For example, quantum dots with CdSe in the core and ZnS in the shell (Aldrich Product Nos. 731838, 731870) available from Aldrich Materials Science exhibit greater than 80% quantum yield. Coating quantum dots with shells improves quantum yield by passivizing nonradiative recombination sites and also makes them more robust to processing conditions for various applications. This method has been widely explored as a way to adjust the photophysical properties of quantum dots.8-10
Alloyed Quantum DotsThe ability to tune optical and electronic properties by changing the crystallite size has become a hallmark of quantum dots. However, tuning the properties by changing the crystallite size could cause problems in many applications with size restrictions. Multicomponent quantum dots offer an alternative method to tune properties without changing crystallite size. Alloyed semiconductor quantum dots with both homogeneous and gradient internal structures allow tuning of the optical and electronic properties by merely changing the composition and internal structure without changing the crystallite size. For example, alloyed quantum dots of the compositions CdSxSe1-x/ZnS of 6nm diameter emits light of different wavelengths by just changing the composition (Aldrich Prod. Nos. 753742, 753793) (Figure 2). Alloyed semiconductor quantum dots formed by alloying together two semiconductors with different band gap energies exhibited interesting properties distinct not only from the properties of their bulk counterparts but also from those of their parent semiconductors. Thus, alloyed nanocrystals possess novel and additional composition-tunable properties aside from the properties that emerge due to quantum confinement effects.11
Figure 2. Photoluminescence of alloyed CdSxSe1-x/ZnS quantum dots of 6nm diameter. The material emits different color of light by tuning the composition.
Quantum Dots ApplicationsThe unique size and composition tunable electronic property of these very small, semiconducting quantum dots make them very appealing for a variety of applications and new technologies.12
Quantum dots are particularly significant for optical applications owing to their bright, pure colors along with their ability to emit rainbow of colors coupled with their high efficiencies, longer lifetimes and high extinction coefficient. Examples include LEDs and solid state lighting, displays and photovoltaics.7,13,14
Being zero dimensional, quantum dots have a sharper density of states than higher-dimensional structures. Their small size also means that electrons do not have to travel as far as with larger particles, thus electronic devices can operate faster. Examples of applications taking advantage of these unique electronic properties include transistors, solar cells, ultrafast all-optical switches and logic gates, and quantum computing, among many others.13-15
The small size of quantum dots allow them to go anywhere in the body making them suitable for different bio-medical applications like medical imaging, biosensors, etc. At present, fluorescence based biosensors depend on organic dyes with a broad spectral width, which limits their effectiveness to a small number of colors and shorter lifetimes to tag the agents. On the other hand, quantum dots can emit the whole spectrum, are brighter and have little degradation over time thus proving them superior to traditional organic dyes used in biomedical applications.16
Aldrich Materials Science offers semiconductor, core-shell and alloyed quantum dots tuned to emit different colors in the visible spectrum while exhibiting high quantum yield. Nanocrystals are available in both aqueous and organic formulations suitable for use in different applications.
Find the complete list of Quantum Dots offered by Aldrich Materials Science here.
References17. Ekimov, A. I.; Onushchenko, A. A. JETP Lett. 1981, 34, 345–349.
18. Kastner, M. A. Physics Today, 1993, 46(1), 24.
19. Ashoori, R. C. Nature, 1996, 379(6564), 413.
20. Collier, C. P.; Vossmeyer, T.; Heath, J. R. Annual Review of Physical Chemistry, 1998, 49, 371.
21. Reimann, S. M.; Manninen, M. Reviews of Modern Physics, 2002, 74(4), 1283.
22. Bawendi, M. C.; Steigerwald, M. L.; Brus, L. E. Annual Review of Physical Chemistry, 1990, 41, 477.
23. Yoffe, A. D. Advances in Physics, 2001, 50(1), 1.
24. Bailey, R. E.; Nie, S. Edited by Rao, C. N. R.; Mueller, A.; Cheetham, A. K. Chemistry of Nanomaterials, 2004, 2, 405.
25. Dorfs, D.; Eychmueller, A. Zeitschrift fuer Physikalische Chemie, 2006, 220(12), 1539.
26. Smith, A. M.; Nie, S. Nature Biotechnology, 2009, 27(8), 732.
27. Vastola, G.; Zhang, Y.-W.; Shenoy, V. B. Current Opinion in Solid State & Materials Science, 2012, 16(2), 64.
28. Vahala, K. J. Nature, 2003, 424(6950), 839.
29. Nirmal, M.; Brus, L. Accounts of Chemical Research, 1999, 32(5), 407.
30. Sargent, E. H. Nature Photonics, 2012, 6(3), 133.
31. Zhao, Y.; Burda, C. Energy & Environmental Science, 2012, 5(2), 5564.
32. Medintz, I. L.; Uyeda, H. T.; Goldman, E. R.; Mattoussi, H. Nature Materials, 2005, 4(6), 435.
- See more at: http://www.sigmaaldrich.com/materials-science/nanomaterials/quantum-dots.html#sthash.rZO3jKbJ.dpuf
A. Photoinitiated Free Radical Polymerization
When polymerizations are initiated by light and both the initiating species and the growing chain ends are radicals, we speak of radical photopolymerization. In far the most cases of photoinduced polymerization, initiators are used to generate radicals. One has to distinguish between two different types of photoinitiators.See animation of the photopolymerization of the different type monomers. Photopolymerization of monofunctional monomers, Photopolymerization of bifunctional monomers
Type I Photoinitiators: Unimolecular Photoinitiators. These substances undergo an homolytic bond cleavage upon absorption of light. Benzoin and its derivatives are the most widely used photoinitiators for radical polymerization of vinyl monomers.
Type II Photoinitiators Bimolecular Photoinitiators. These photoiniating systems consist of a photoinitiator such as benzophenone or thioxanthone and a coinitiator such as alcohol or amine. Radicals are generated in a bimolecular process by the reduction of photoexcited aromatic carbonyl compound by hydrogen abstraction or electron transfer reactions
B. Photoinitiated Cationic Polymerization
Direct Acting Systems
Upon photolysis, these thermally and hygroscopically stable initiators undergo irreversible photofragmentation to produce cation radicals and Brønsted acids. Reactive species thus produced photochemically with onium salts initiate the cationic polymerization of suitable monomers as illustrated below.
Indirect Acting Systems
For practical applications, onium salts should absorb light appreciably at wavelengths longer than 350 nm where the commercially available medium and high-pressure mercury lamps emit much of their radiation. The indirect actions of these systems, which extend their spectral sensitivity to longer wavelengths, are shown below..
Several indirect ways to expand wavelength range have been described. All of these pathways involve (i) electron transfer reactions either with photoexcited sensitizer or free radicals (iii) and with the electron donor compounds in the excited charge transfer complexes (ii)
(i) Photosensitizer
Many aromatic hydrocarbons such as anthracene, phenothiazine, and perylene are able to sensitize the decomposition of onium salts via electron transfer. The irradiation of the sensitizer is followed by the formation of a complex between excited sensitizer molecules and ground state onium salt. In this complex, one electron is transferred from the sensitizer to the onium salt giving rise to the generation of sensitizer radical cation as a result of homolytic cleavage of the corresponding onium salt. The radical cations themselves initiate the polymerization of appropriate monomers or, alternatively, interact with hydrogen donor constituents of the polymerization mixture (such as solvent or monomer) resulting in the release of Brønsted acid.
(ii) Charge Transfer Complex
Pyridinium salts are capable of forming charge transfer (CT) complexes with electron rich donors such as methyl- and methoxy-substituted benzene. It was found that the CT complexes formed between pyridinium salts and aromatic electron donors act as photoinitiators for the cationic polymerization of cyclohexene oxide and 4-vinyl cyclohexene oxide. The mechanism illustrated in equations 19 and 20 for the initiation of the cationic polymerization has been suggested.
(iii) Free Radical Promoted Cationic Polymerization
Among the indirectly acting initiating systems, free radical promoted cationic polymerization is the most flexible route, since free radical photoinitiators with a wide range of absorption characteristics are available. Many photochemically formed radicals can be oxidized by onium salts. The cations thus generated are used as initiating species for cationic polymerization according to the following reactions.
[F20]They are molecules composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube.Spherical fullerenes are sometimes called buckyballs, the C60 variant is often compared to a typical white and black soccer football.
Cylindrical fullerenes are called buckytubes.
Recently discovered is the "buckyegg", by researchers at UC Davis.
Fullerenes are similar in structure to graphite, which is composed of a sheet of linked hexagonal rings, but they contain pentagonal (or sometimes heptagonal) rings that prevent the sheet from being planar.
[F21]Dendrimers are highly branched, star-shaped macromolecules with nanometer-scale dimensions. Dendrimers are defined by three components: a central core, an interior dendritic structure (the branches), and an exterior surface with functional surface groups. The varied combination of these components yields products of different shapes and sizes with shielded interior cores that are ideal candidates for applications in both biological and materials sciences. While the attached surface groups affect the solubility and chelation ability, the varied cores impart unique properties to the cavity size, absorption capacity, and capture-release characteristics. Applications highlighted in recent literature include drug delivery, gene transfection, catalysis, energy harvesting, photo activity, molecular weight and size determination, rheology modification, and nanoscale science and technology.1, 2 Monodisperse dendrimers are synthesized by step-wise chemical methods to give distinct generations (G0, G1, G2, ...) of molecules with narrow molecular weight distribution, uniform size and shape, and multiple (multivalent) surface groups Z. Dendrons are monodisperse wedge-shaped sections of dedrimers with a single focal point reactive function. Hyperbranched polymers are polydisperse dendritic macromolecules synthesized by lower-cost polymerization methods.
(1) Functional Polymers and Dendrimers: From Synthesis to Application.
Proceedings of the American Chemical Society Division of Polymeric Materials: Science & Engineering, San Diego, CA, April 1-5, 2001; ACS, 2001
(2) Dendrimers and Other Dendritic Polymers, Jean M.J. Frechet and Donald A. Tomalia, J. Wiley & Sons (2001)
- See more at: http://www.sigmaaldrich.com/materials-science/material-science-products.html?TablePage=16375655#sthash.Vk362d8T.dpuf
[F22]This is what makes this such a controversial reality with this technology is how it activates and reacts and how it can literally take over
[F23]Understanding the Volume Ratio and the Smaller the size the bigger the volume of nano which means just the materials themselves can oxidize or choke out cells and tissues in any life form add a chemical to this in any form and you overload the essences of life and with the size factor will alter or change or over write any environment it is relseased into
[F24]Exposure to Nano particles comes in 3 ways
[F25]This would explain as well pathologies that occur in different parts of the body as well as secondary pathogens occurring in the system due to the nanopollutants as well as the nano engineering that can go on once these nanos are programmed
[F26]3 categories of exposure > 2.5 mm-< 2.5 mm < 100nm
[F27]Micron Particles > 2,5 can be excreted by several different means
[F28]Micron particles < 2.5 are deeper into the lung cavity and there is where white blood cells remove ( provided they can break down the material if not then the material wil embed especially if in NM range)
[F29]Entry of nano throughout the system
[F30]The increase toxicity of Nanoplatelets~ can penetrate skin and respiratory system
[F31]The term Micro is different then Nano and micro will not pass where nano will
[F32]Pay attention to the food and drink part especially for those who are not working in the industry this is an indicator that the food and beverages will have this in them
[F33]More Research has come out showing organ damage as a result of accumulation and area and volume and density of the nano ~ due to it’s accumulation and the system is not able to clear it out fast enough to get everything back up where it should be
[F34]The Finer the dust the volatile the effect
[F35]The Fire Power of Nano with an Aluminum mix
[F36]Atmospheric Particles could provide a route for toxic or reactive NanoParticle in the food chain
[F37]Non POINT source ~ how about chemtrails???or glyphosate and atrazine sprays in the crops~ how about Vaccines~ Water
[F38]Internalized = active or used or integrate
[F39]Nanoparticles gets through Clothing~ so there is little protection from outer exposure