[0028] In this example, an ATP synthesis activator was prepared by mixing herbs in
Thyme----10% by weight
Rosemary--10% by weight
Turmeric--10% by weight
Fennel--15% by weight
Grape seeds--15% by weight
Dandelion--15% by weight
Acanthopanax senticosus (Substitute Ginseng-Siberian or even Rhodiola)-- 30% by weight
All are powdered and then either made into a Pill form or encapsulated—could be fused as well in alcohol or other mixes to increase the usage and methods
*******************************************************************
Formula 2 ATP Solution
An aqueous solution containing the following components was made:
10 mg/ml of adenosine 5′-triphosphate disodium salt (anhydrous) (18.15 mM)
1 mg/ml of glycine (13.32 mM) –(Substitute Creatine)
36 mg/ml of Na2HPO4.7H2O (134.3 mM) ( Substitute TSP)
Sodium hydroxide (sufficient to adjust pH to 9.2-9.3)
Water sufficient to result in the above concentrations of adenosine 5′-triphosphate disodium salt (anhydrous), glycine, and 36 mg/ml of Na2HPO4.7H2O.
Basically – 100 ml – of water
ATP- 1 gram---Creatine 100mgs—3.6 grams of TSP- ( also as addition add edta 1 gram and glycerol as well 10mls)
Repeat the formula and add edta 1gram—calcium carbonate ( or any other form) Potassium chloride or citrate-and about 100grams of sunflower lecithin ( you can add other ATP producers like ginger and rhodiola as we in either powder or extract) mix altogether and when it solidifies you have a liposomal ATP mix
ATP, ADP, NAD+5 6
Exogenous substances that increase endogenous production of ATP are:
ATP ATP 100 (ATP), Chromium Polynicotinate (Glucose Tolerance
Factor) (HEED, Sustained Energy, Perpetuem, Premium Insurance Caps),
Acetyl-L-Carnitine (Mito-r-Caps), LCarnitine (Sustained Energy &
Perpetuem), Ginkgo Biloba (Super AO), Magnesium (Endurolytes & Premium
Insurance Caps), Inosine (Race Caps Supreme), Coenzyme Q-10 (Race Caps
Supreme), Vinpocetine (Super AO), Vitamin B3 (Premium Insurance Caps),
Vitamin B5 (Premium Insurance Caps), and Vitamin C (Premium Insurance
Caps).Undetermine
d7
ENZYME ACTIVATORS TIMED DOSE FINAL PORTION OF WORKOUT
Coenzyme Q-108 9, Idebenome 10 (Race Caps Supreme) 30-60 mg GENE EXPRESSION INCREASE IN ATP-ENERGY INCREASE IN PERFORMANCE
Coenzyme Vitamin B-211 12 13 (Premium Insurance Caps) 100-200 mg INCREASE IN PERFORMANCE FADH2
5 Glucose, Chromium Polynicotinate (Glucose Tolerance Factor), Carnitine, Acetyl-L-Carnitine (ALC), LCarnitine,NADH, Ginkgo Biloba, Triiodothyronine (T3), Magnesium, Inosine, Coenzyme Q10, Hydergine,Piracetam, Vinpocetine, Vitamin B3, Vitamin B5, Vitamin C increase the endogenous production of ATP.(Dean, Vitamin Research News. May 2000; Nicholson, Psychopharm. 101:147-59, 1985; Kisters et al. Magnes Res.
13:183-8, 2000; Regelson & Goodman, Thyroid Hormone. In: The Super-Hormone Promise: Nature’s Antidote to Aging. Simon & Schuster, New York, NY, USA, 1996:169; Sinatra et al. L-Carnitine and the Heart. Keats Publishing. Los Angeles, USA. 1999:9; Seifulla et al. Exp Clin Pharmacol. 56(6):34-36, 1993).
Adenosine Triphosphate
is an endogenous compound that contains Adenine + Ribose (i.e. Adenosine) and three Phosphate groups. ADP is the precursor for the endogenous production of Adenosine Triphosphate (ATP). Adenosine Diphosphate is an endogenous substance manufactured within the body as part of the krebs cycle of energy production. Endogenous ATP is produced by oxidation of various chemicals the Krebs Cycle and is the body's primary "storehouse" of cellular energy. The human body is constantly manufacturing (and breaking down) ATP for
Energy production. Glucose is one of several precursors for the production of ATP - one molecule of Glucose can generate 38 molecules of ATP. Also the Electron Transport System (ETS) accepts hydrogen atoms via the Krebs cycle and passes them through a series of compounds until they combine with oxygen to form water. Each cycle of this process produces 11 molecules of ATP. The total amount of ATP recycled via metabolization by the average person for energy amounts to approximately 150-187 lbs of ATP each day. If deficits occur, fatigue results. Optimal peak production of ATP is required in order to avoid fatigue. One of the underlying causes of Chronic Fatigue Syndrome is impaired production of mitochondrial Adenosine Triphosphate (ATP). CFS patients have elevated
blood levels of lactic acid, indicating suboptimal aerobic ATP production that can lead to fatigue and muscle aches. 7 “Undetermined” indicates the papers reviewed did not conclude the dosage range for affects..
Coenzyme Q10 retards the aging process (including the aging process within the brain). Aging is associated with a decrease in coenzyme Q10 levels inside and outside mitochondria. (Lenaz et al.Biofactors. 8:195-204, 1998)
11 Vitamin B2 protects the energy-producing mechanisms of the body from free radicals damage. · Haas, R. Smart antioxidants for your mind. In: Eat Smart Think Smart. Harper Collins Publishers. 1994:82.
Coenzyme Vitamin B-3 100-200 mg INCREASE IN PERFORMANCE NADH ACEYTL L-CARNITINE (OR LCARNITINE- 750-1000 mg GENE EXPRESSION INCREASE IN ATP-ENERGY
r-ALPHA-LIPOIC ACID 500-750 mg GENE EXPRESSION INCREASE IN ATP-ENERGY
N-ACETYL CYSTEINE 600-1200 mg INCREASE IN RECOVERYRATE
& ATP-ENERGY Hendler, Sheldon Saul. The Doctor’s Vitamin and Mineral Encyclopedia. Arrow Books, London, England 1991:49. 9 Heart levels of Coenzyme Q10 decline with the progression of the aging process. Coenzyme Q10 has been found to reverse accelerated apoptosis of cells involved in the aging process (this means that coenzyme Q10 has potential as a life extension agent by retarding the accelerated death of cells involved in the aging process). (Coles & Harris, Coenzyme Q-10 and life extension. In: Advances in Anti-Aging Medicine. Volume 1. Dr. Ronald M. Klatz (editor),1996)Kagan et al. Annals of the New York Academy of Sciences 887:31-47, 1999)
10 Idebenone is a smart drug that is similar in chemical structure to Coenzyme Q10 (a Quinone) and when present simultaneously enhances the potency of Coenzyme Q-10. Idebenone prevents Free Radicals from damaging the Deoxyribonucleic Acid (DNA) content of the Mitochondria (mitochondrial DNA). Idebenone is reported to enhance the function of and protect the mitochondria. (Wieland et al. Transplantation; Mason Anti-Aging Bulletin. 4(4):22-25, 1999)
12 Vitamin B2 facilitates the metabolic production of energy from foods. Vitamin B2 plays a major role in the conversion of carbohydrates, proteins and fats into forms that the body can use for the production of energy. Mervyn, L. Thorsons Complete Guide to Vitamins and Minerals (2nd Edition). Thorsons Publishing
Group, Wellingborough, England. 1989:39. Roberts, A. J., et al. Nutraceuticals: the Complete Encyclopedia of Supplements, Herbs, Vitamins and Healing Foods. Berkely Publishing Group. New York, USA. 2001:225.
13 Vitamin B2 is a precursor for the production of Flavin Mononucleotide (FMN) and Flavin Adenine Dinucleotide (FAD), which are Hydrogen carriers for the production of Adenosine Triphosphate (ATP) within the body. Studies
have confirmed that people who engage in regular exercise have a higher daily requirement for vitamin B-2. 14 Haas (1992) reports that decreased energy production is one of the first signs of vitamin B3 deficiency. Haas, Elson M. Staying Healthy with Nutrition. Celestial Arts, Berkeley, California, USA. 1992:119.
15 Vitamin B3 is involved in the metabolism of carbohydrates. Vitamin B3 is essential for the body's production ofEnergy (due to its role in the manufacture of Adenosine Triphosphate (ATP). Vitamin B3 reduces fatigue.
Carnitine (acetyl-l and l- forms) resides in the cytoplasm of cells where it combines with molecules of Acetyl Coenzyme A and molecules of long-chain saturated fatty acids to form a complex that can penetrate the wall of the
mitochondria and facilitates the transportation of fatty acids across cell membranes into the mitochondria. Carnitine in the brain, blood, heart and muscles decreases in tandem with age, while carnitine levels in the liver increase with
the aging, indicating that the transportation of carnitine from the liver to the blood declines as we age. Carnitine retards the progression of the aging by preventing cell death via the maintenance of energy supply to individual cells.
(Maccari et al. Exp Gerontol. 25:127-134, 1990; Paradies et al. Mech Ageing Dev. 84(2):103-112, 1995.)
17 Carnitine (acetyl-l and l- forms) increases the body's production of energy. Carnitine facilitates the transport of long-chain saturated fatty acids into the mitochondria and stimulates the oxidation of fatty acids to produce energy. A
molecule of carnitine in the cytoplasm outside the mitochondria combines with a molecule of fatty acid and a molecule of Acetyl Coenzyme A to make a complex that can penetrate the wall of the mitochondria. Inside the mitochondria the complex liberates the carnitine, which can move outside to repeat its action of serving as a shuttle to carry more molecules into the mitochondria. (Arenas et al. Muscle & Nerve. 14(7):598-604, 1991.)
18 Carnitine improves cardiovascular function in people who engage in exercise, reduces the accumulation of lactic acid during exercise, prevents muscle pain in people who exercise, preserves muscle glycogen during exercise, increases muscle carnitine levels during exercise, prevents exercise-induced declines in muscle carnitine levels and increases the rate of recovery following exercise. (Brass et al. J Am Coll Nutr. 17:207-215, 1998; Bremer, Physiol Rev. 63:1420-1480, 1983; Cerretelli et al. Int J Sports Med. 11(1):1-14, 1990.)
Citrate, cis-Aconitate,Isocitrate,Oxalosuccinate,alpha-Ketoglutarate,Succinyl-CO-A,
Succinate,Fumarate,Malate.(Race Caps Supreme’s d-AlphaTocopherol Succinates, Alpha-Ketoglutarate, proprietary kreb cycleintermediates)Undetermine d20
KREBS CYCLE INTERMEDIATES
Alpha-Ketoglutarate, Glycine, Alanine.(Race Caps Supreme’s Alpha-
Ketoglutarate & proprietary kreb cycle intermediates; Glycine is formulated in HEED & Endurolytes powder.) Undetermined 21 SPECIFIC PROTEIN ACTIVES
KREB CYCLE & OXIDATIVE PHOSPHORYLATION
Quercetin (3,5,7,30 ,40 -Pentahydroxyflavone) 22 23 24, 30-50 mg GENE EXPRESSION,INFLUENCE = INCREASE INRECOVERY-RATE & ATPENERGY Dimethylaminoethanol (DMAE) 25 26 27 28 -500-100 mg GENE EXPRESSION INFLUENCE = INCREASE IN RECOVERY-RATE & ATPENERGY
Carnosine 29 30 (HEED, Sustained Energy, Perpetuem) 50 mg REDUCE AGE’s ENERGY RECOVERY-RATE & ATP ENERGY
BIOTIN 31
MITOCHONDRIA/GENE EXPRESSION EFFECTS ENZYME PRECURSORS
metabolism; gene expression VIA glucokinase synthesis
Hexokinase,
Glucose-Phosphate Isomerase,
Adolase,
Triosephosphate-Isomerase,
Glyceraldehyde 3-Phosphate-
Dehydrogenase, Phosphoglycermutase
Enolase,
Pyruvate Kinase.
(Race Caps Supreme’s & Premium
Insurance Caps’ Enzyme Enhancement
Undetermine d32
Cysteine food sources include whey protein isolates, poultry, yogurt, egg yolks, red peppers, garlic, onions,. Whey proteins are rich in cysteine as well as in GSH precursor peptides. Recent clinical trials have demonstrated that intake of cysteine-rich whey protein formulas benefits patients with HIV/AIDS. In both short-term (2 weeks) and long-term (6 months) studies, supplementation with whey protein formulas increased plasma glutathione levels in patients with HIV infections. Further, the treatment was well tolerated. Also, intake of a cysteine-rich whey protein supplement for eight weeks increased weight gain, reduced the occurrence of gastrointestinal side effects, and improved tolerance to highly active anti-retroviral therapy in HIV patients (Pacheco et al., J. Human Virology 5(1), 2002). 20 “Undetermined” indicates the papers reviewed did not conclude the dosage range for affects.
Quercetin was demonstrated to reduce (inflammation) the concentration of prostaglandin E2 in the pleural exudates of rats given carrageenan intrapleurally. (Mascolo, N., et al. J Pharm Pharmacol. 40:293-295, 1988.) 23
Quercetin has been demonstrated to inhibit (inflammation) mast cell degranulation and the subsequent release of histamine. (Miller, A. Alternative Medicine Review. 6(1):20-47, 2001.)
Quercetin protects the body’s endogenous Deoxyribonucleic Acid (DNA) from breakage from ferric iron-initiated hydrogen peroxide lipid peroxidation (Quercetin removes ferric iron out of the body). (Duthie et al. European Journal of Nutrition. 38(1):28-34, 1998.) 25 Pieralisi reports that athletes taking
DMAE for only 6 days significantly improved total workload capacity and
maximal aerobic capacity (VO2 max). (Pieralisi et al. Clin Ther.13(3):373-382, 1991.) 26 Hochschild investigated the potential life-extending effect of
DMAE on old mice. DMAE administration in the drinking water resulted in a reduction in mortality and an increase in both mean and maximum survival times. This research found that DMAE extended the lifespan of mice by 27-49%. (Hochschild. Exp Geront. 8(4):177-183, 1973.)
DMAE increases energy and reduces fatigue. (Pfeiffer et al. Science. 126:610-611, 1957.)
DMAE inhibits (and reverses) the cross-linking of endogenous proteins. DMAE diminishes the extent of crosslinking in aged rats. (Zs-Nagy et al. Mech Aging Dev. 14:245-251, 1980.
Carnosine (beta-alanyl-L-histidine) rejuvenates cells that have reached their "Hayflick Limit" (causing the senescent phenotype of old cells that have reached their hayflick limit to revert to their juvenile phenotype state). The
Hayflick Limit is the point at which a Cell reaches Cellular Senescence. Most Cells regenerate themselves by dividing to form two new Cells. Eventually Cells reach a limit beyond which they can no longer continue to divide. Senescent Cells that have reached their Hayflick Limit do not die, however they do become distorted in their form and function. For example, cultured human Fibroblasts have a Hayflick Limit of 60-80 divisions. Cells that have reached their Hayflick Limit have a grainy appearance. They assume odd sizes and shapes and no longer line up in
parralel arrays. These changes are known as the senescent phenotype. (Hipkiss et al. Perspect Hum Biol. 1:59-70, 1995 & Yuneva et al. J Anti-Aging Medicine. 2:337-342, 1999.)
Carnosine (beta-alanyl-L-histidine) has protective functions additional to antioxidant and free radical scavenging roles. It extends cultured human fibroblast life span, kills transformed cells, protects cells against aldehydes and an
amyloid peptide fragment and inhibits, in vitro, protein glycation (formation of cross-links, carbonyl groups and AGEs) and DNA/protein cross-linking. Carnosine is an aldehyde scavenger, a likely lipofuscin (age pigment)
precursor and possible modulator of diabetic complications, atherosclerosis and Alzheimer's disease. (Boldyrev et al.
Biosci Rep. 19 (6), 581-587, 1999 & Hipkiss Int J Biochem Cell Biol. 30(8):863-868, 1998.)
31 Biotin deficiency is common. Estrogen excess, caffeine, egg whites, alcohol (ethanol) reduce or destroy the body's reserves of biotin. Pharmaceutical antibiotics destroy the beneficial intestinal bacteria that produce endogenous biotin and some forms of also directly destroy biotin itself. As deficiency occurs, reduced energy and premature fatigue are predicted. Biotin is closely involved (via its role as a cofactor for various enzymes) in the endogenous production of
energy and the process of gluconeogenesis. Gluconeogenesis is a metabolic process involving in the formation of glucose by the liver from non- carbohydrate precursors such as glycerol, lactic acid or glucogenic amino acids. With
Biotin deficiency fatigue will result. Four normal subjects were placed on an experimental diet that was deficient in biotin. After ten weeks of this diet, subjects experienced fatigue. Fatigue was relieved by subsequent biotin supplementation. (Sydenstricker et al. JAMA 118:1199-1200, 1940.)
32 “Undetermined” indicates the papers reviewed did not conclude the dosage range for affects.
SystemTM is a proprietary blend of enzymes: protease, amylase,
glucoamylase, lipase, cellulase, phytase, maltase, and sucrase)
Inositol Hexaphosphate (IP6) 33 34 35 36-cDNA micrOanalysis
downmodulatES gene transcription and cell cycle regulation &
coherent upregulation of cell cycle inhibitors REDUCING RISK OF
CARDIOVASCULAR DISEASE/CANCER
Arginine-Lysine-Glucose-ALG 500-1000 mg each
post workout ADDITIVE carbohydrates to prevent AGE's
REDUCE AGE’s INCREASE RECOVERY-RATE & ATP ENERGY
HYDERGINE PRECURSORS 6-9 mg as Hydergine GENE EXPRESSION INCREASEIN ATP-ENERGY
Urticaria Tomentosa (Cat’s claw) 38 39 20-60 mg Lipopolysaccharide-induced iNOS gene expression, nitrite formation, cell death and inhibited the activation of NF-kappaB ANTIOXIDANT & ANTIINFLAMMATORYAGENT
FORSKOLIN 40 41 250-500 mg unique diterpene activator of camp, specific stimulator of adenylyl cyclase, inhibits the release of histamine, GENE
EXPRESSION INFLUENCE = circadian gene expression (rPer1, rPer2, dbp)
INCREASE IN RECOVERYRATE & ATP-ENERGY
Inositol Hexaphosphate is an organic acid composed of one molecule of the myoinositol form of inositol joined with six phosphorus molecules (i.e. It is the hexaphosphate ester of inositol). IP6 up-regulates the expression of the
tumor suppressor gene p53 and p21WAF1/CIP1 gene and their modulation may be one of the mechanisms of the anti-neoplastic action of IP6. Since loss of p53 function enhances cancer cells' resistance to chemotherapeutic agents,
the stimulating function of IP6 on p53 makes it an attractive (anti-cancer) adjuvant chemotherapeutic agent. (Saied et al. Anticancer Research. 18(3A):1479-1484, 1998)
34 Inositol Hexaphosphate prevents various types of cancer by repairing damaged double-strand DNA breaks Deoxyribonucleic Acid (DNA). (Hanakahi et al. Cell. 102:721-729, 2000.
35 Inositol Hexaphosphate inhibits the ability of iron to induce lipid peroxidation. (Porres et al. Proc Soc Exp Biol Med. 221(1):80-86, 1999)
36 Inositol is an antioxidant, scavenges hydroxyl free radicals, prevents fatty liver, and may be required for the metabolism of cholesterol and elevated serum cholesterol can occur as a result of inositol deficiency. (Ramakrishnan
et al. Indian J Biochem Biophys. 36(2):129-33, 1999)
37 The structure of a synthetic glucose-arginine-lysine derived advanced glycation end product that is immunologically cross-reactive with naturally occurring counterparts. The properties of arginine-lysine imidazole
(ALI), immunoreactivity, acid-lability, nonfluorescence, and inhibition of formation by aminoguanidine, suggest that ALI is likely to typify an important class of the AGE cross-links that form in vivo. (Al-Abed & Bucala. Bioconjug
Chem. 2000 Jan-Feb;11(1):39-45)
Cat’s claw (Uncaria tomentosa) is a medicinal plant from the Amazon River basin, widely used for treating inflammatory disorders. It has previously been described as being an inhibitor of NF-kappaB. Cat’s claw is an
effective antioxidant and is a potent inhibitor of TNFalpha production. (Aquino et al. J Nat Prod. 54(2):453-459, 1991; Sandoval et al. Free Radical Biology & Medicine. 29(1):71-78, 2000)
Uncaria tomentosa water extracts (C-Med-100) was shown to enhance DNA repair, mitogenic response and leukocyte recovery after chemotherapy-induced DNA damage in vivo. (Sheng et al. Phytomedicine. 8(4):275-282,
2001)
GOAT’S RUE (G. Officinalis) 42 43 200-300 mg Guanidine-INDUCED
hypoglycemic EFFECTS, positive effects on insulin receptorexpression & tyrosine kinase activity, pharmacological inhibitor of iNOS, NE, & ACh REDUCED
PROSTGLANDIN PRODUCTION
Butein (3,4,20 ,40 - Tetrahydroxychalcone)44 50 mg GENE EXPRESSION
INFLUENCE = INCREASE IN RECOVERY-RATE & ATPENERGY
Piceatannol (3,5,30 ,40 -tetrahdroxytrans- Stilbene)45 30-50 mg GENE EXPRESSION INFLUENCE = INCREASE IN RECOVERY-RATE & ATPENERGY
40 Forskolin enhances Energy production by every Cell in the body (due to it elevating the body’s cAMP levels) and
assists the treatment of hypothyroidism by stimulating the production and release of thyroid hormones. (Haye et al.
Mol Cell Endocrinol. 43:41-50, 1990; Roger et al. Exp Cell Res. 172(282-290), 1990; Saunieret al. J Biol Chem.
265:19942-19946, 1990.)
41 Forskolin facilitates weight loss in persons afflicted with obesity by stimulating the production of camp, which in turn regulates the process of lipolysis. Forskolin inhibits the endogenous synthesis of fatty acids in adipocytes. Forskolin counteracts the decreased response by adipocytes to adrenaline, which occurs as a result of the aging process. (Allen et al. J Pharhacol Exp Ther. 238(2):659-664, 1986; Ho et al. Biochem Biophys Res Commun. 107(1):157-164, 1982; Hoffman et al. Horm Metab Res. 19:358-360, 1987; Okuda et al. J Lipid Res. 33:225-231,
1992.)
42 Goat’s Rue lowers elevated blood sugar (glucose) levels (due to the Galegine content of goat’s rue). Goat’s Rue helps to prevent cross-Linking (it prevents the formation of toxic glycosylation end-products. Goat’s Rue contains Galegine (2-methyl-2-butenyl-guanidine) a raw material used in the manufacture of pharmaceutical drugs such as
Aminoguanidine, Metformin, and Phenformin. Petricic et al found that Goat’s rue lowered blood sugar in diabetic rats by 32%. Muller reported that Goat’s Rue lowers blood sugar in diabetes mellitus patients and in normal subjects.
(Petricic et al. Acta Pharm Jugosl. 32(3):219-223, 1982; Muller et al. Arch Expll Path Pharm. 125:212-228, 1927.)
43 Substances in Goat’s Rue are used as precursors for the production of Aminoguanidine. However, both Vitamin B-1 & Carnosine are more potent substances for preventing and reversing cross-linking compared to
Aminoguanidine.44 Research reports three classes of small molecules that activate sirtuins. They show that the potent activator
resveratrol, a polyphenol, lowers the michaelis constant of sirt1 for both the acetylated substrate and NAD 1, and increases cell survival by stimulating sirt1- dependent deacetylation of p53. In yeast, resveratrol mimics calorie
restriction by stimulating sir2, by increasing DNA stability and extending lifespan by 70%, and based on this research, I estimate that stimulation of sirti catalytic rate by plant polyphenols compound ratio to control
Butein by 45%, Piceatannol by 41%, Isoliquiritigenin by 39%, Fisetin by 34%, Quercetin by 24%. (Sinclair et al., Small molecule activators of Sirtuins extend saccharomyces cerevisiae lifespan. Nature. 2003 sep 11;425(6954):191-6. Epub 2003 aug 24.)
45 Piceatannol is a naturally occurring analog of Resveratrol. Piceatannol is a closely related stilbene that has antileukaemic activity and is also a tyrosine kinase inhibitor. Piceatannol differs from resveratrol by having an additional aromatic hydroxy group. The enzyme CYP1B1 is overexpressed in a wide variety of human tumours and catalyses aromatic hydroxylation reactions. We report here that the cancer preventative agent resveratrol undergoes metabolism by the cytochrome P450 enzyme CYP1B1 to give a metabolite, which has been identified as the known
antileukaemic agent piceatannol. Piceatannol can be considered to be a promising chemopreventive or anticancer agent. (see footnote 11) Piceatannol increases cell lifespan by 41% compared to 70% for its parent metabolite Resveratrol.
RNA:DNA 10:1 50 1500-1800mg agE increase in cell rRNA INADEQUATE FOR cell volume
PROPIONYL L-CARNITINE51 Sublingual 200 mg x 1- INCREASE ATP STORE &
ENERGY
49 Resveratrol is found in grape skins, seeds, stalks, vines and roots, but not the flesh of the fruit. The highest concentration of Resveratrol is in the grape skins. Resveratrol content of grape skins is 50-100 mcg per gram or 5,000-10,000 mcg per 100 grams. While I was excited to read of David Sinclair's research that reported that Resveratrol advanced lifespan, it also may produce mild estrogenic effects that oppose the androgenic value of testosterone particularly in males, partially in females. Until more research answers the safety and efficacy questions
about this interesting substrate, I chose to reserve all testing applications.
Genetic regulator of lifespan identified a gene that extends lifespan in yeast points to paradigm shift in longevity research may explain life extension via calorie restriction. Researchers at Harvard Medical School (HMS) discovered
that a gene that may control lifespan. The gene, PNC1, determines lifespan according to its biochemical environment. A yeast strain with five copies of PNC1 lives 70 percent longer than other strains. The PNC1 gene protein a form of
vitamin B3. Vitamin B-3 inhibits Sir2 gene expression and may either lengthen or shorten lifespan. Under normal conditions, Sir2 uses NAD as a cofactor to produce nicotinamide, which then inhibits Sir2 in a negative feedback loop. When the cell is exposed to environmental stresses like calorie restriction, heat shock, or osmotic stress (top), PNC1 is turned on. Then, Pnc1 protein converts nicotinamide to nicotinic acid, a molecule with no effect on Sir2. No longer inhibited by nicotinamide, Sir2 is inactivated resulting in increased cell lifespan. Sir2 extends lifespan by keeping ribosomal DNA stable. PNC1 converts nicotinamide into nicotinic acid, a molecule that does not affect lifespan. In doing so, it keeps nicotinamide from inhibiting Sir2, allowing the cells to live longer. The finding implies that lifespan is not simply dependent on accumulated wear and tear or metabolism, as some have suggested, but is at least partly controlled by an active genetic program in cells--one that could theoretically be boosted. Life extension
from calorie restriction is a result of an active cellular defense involving turning on a specific gene. Severe calorie restriction extends the lives of many organisms like yeast, fruit flies, worms, and rats, and slows the aging process even preventing cancer in rats. While Sir2 is a necessary part of the equation, calorie restriction does not affect Sir2 levels, indicating that Sir2 must be regulated by another protein that does respond to calorie restriction. NAD, a cofactor of Sir2 and metabolite in the cell regulates metabolism. Because NAD level correlates to metabolism rate, this suggests that calorie restriction might lengthen lifespan by lowering metabolism. Dr. Sinclair's group showed that the effect of PNC1 was independent of NAD availability. They believe that the real regulator of Sir2 is
nicotinamide, which is one of the products of the reaction between Sir2 and NAD. PNC1 levels are highly sensitive to environmental cues like calorie restriction, low salt, and heat that are known to make yeast live longer. Sinclair's team
believes that the PNC1/nicotinamide pathway provides a genetic link between the environment of an organism and its lifespan, allowing an organism to actively change its survival strategies according to the stress sensed. Humans however have seven Sir genes, not just Sir2. The nicotinamide pathway inhibits human SIRT1, a homologue of Sir2. One of the immediate implications of the work is that it emphasizes the functional difference between nicotinamide and nicotinic acid. Nicotinic acid (niacin) is a known anticholesterol treatment, while nicotinamide (or niacinimide) is sometimes touted for anti-aging abilities and is in clinical trials as a therapy for diabetes and cancer. Sinclair's study raises the concern of taking high doses of nicotinamide," Sinclair said, because nicotinamide puts a damper on Sir2's
actions in the cell.50 Supplemental RNA or DNA is broken down (metabolized) within the body to its constituent Purines (Adenine and Guanine) and Pyrimidines (Cytosine and Uracil). These constituents are then reassembled within the body to form new Ribonucleic Acid (RNA) and Deoxyribonucleic Acid (DNA). Supplemental RNA possibly counteracts the progression of the aging. The Brain’s levels of RNA decline in tandem with age. Supplemental RNA possesses
possible life extension properties. Rats injected with supplemental RNA in conjunction with supplemental DNA lived for twice as long as controls and rats supplemented with oral RNA (25 mg per day) increased their lifespan by +16%.
(Heyden, Nature. 184:433, 1959; Odens, Journal of the American Geriatrics Society. 21:450-451, 1973; Frank, Nucleic Acid and Antioxidant Therapy of Ageing and Degeneration. New York: Rainstone Publishing, 1977.)
***********************************************************************
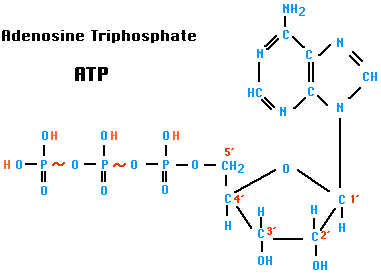
ATP (Adenosine triphosphate)
ATP is a nucleotide that performs many essential roles in the cell.
- It is the major energy currency of the cell, providing the energy for most of the energy-consuming activities of the cell.
- It is one of the monomers used in the synthesis of RNA and, after conversion to deoxyATP (dATP), DNA.
- It regulates many biochemical pathways.
Energy
When the third phosphate group of ATP is removed by hydrolysis, a substantial amount of free energy is released. The exact amount depends on the conditions, but we shall use a value of 7.3 kcal per mole.
ATP + H2O → ADP + Pi
ADP is adenosine diphosphate. Pi is inorganic phosphate. [structure]
Because of the substantial amount of energy liberated when it is broken, the bond between the second and third phosphates is commonly described as a "high-energy" bond and is depicted in the figure by a wavy red line. (The bond between the first and second phosphates is also "high-energy".) (But please note that the term is not being used in the same sense as the term "bond energy". In fact, these bonds are actually weak bonds with low bond energies.)
Cells contain a wide variety of enzymes — called ATPases — that catalyze the hydrolysis of ATP and couple the energy released to particular energy-consuming reactions in the cell (see examples below).
Synthesis of ATP
- ADP + Pi → ATP + H2O
- requires energy: 7.3 kcal/mole
- occurs in the cytosol by glycolysis
- occurs in mitochondria by cellular respiration
- occurs in chloroplasts by photosynthesis
Consumption of ATP
ATP powers most of the energy-consuming activities of cells, such as:
- Most anabolic reactions. Examples:
- joining transfer RNAs to amino acids for assembly into proteins [Link]
- synthesis of nucleoside triphosphates for assembly into DNA and RNA
- synthesis of polysaccharides
- synthesis of fats
- active transport of molecules and ions
- nerve impulses
- maintenance of cell volume by osmosis
- adding phosphate groups (phosphorylation) to many different proteins, e.g., to alter their activity in cell signaling.
- muscle contraction
- beating of cilia and flagella (including sperm)
- bioluminescence
Extracellular ATP
In mammals, ATP also functions outside of cells. Its release
- from damaged cells can elicit inflammation and pain;
- from the carotid body signals a shortage of oxygen in the blood;
- from taste receptor cells triggers action potentials in the sensory nerves leading back to the brain;
- from the stretched wall of the urinary bladder signals when the bladder needs emptying.
********************************************************************
ATP: The Perfect Energy Currency for the Cell
CRSQ Volume 36(1) June 1999
Abstract-
The major energy currency molecule of the cell, ATP, is evaluated in the context of creationism. This complex molecule is critical for all life from the simplest to the most complex. It is only one of millions of enormously intricate nanomachines that needs to have been designed in order for life to exist on earth. This molecule is an excellent example of irreducible complexity because it is necessary in its entirety in order for even the simplest form of life to survive.
Introduction
In order to function, every machine requires specific parts such as the screws, springs, cams, gears, and pulleys. Likewise, all biological machines must have many well-engineered parts to work. Examples include units called organs such as the liver, kidney, and heart. These complex life units are made from still smaller parts called cells which in turn are constructed from yet smaller machines known as organelles. Cell organelles include mitochondria, Golgi complexes, microtubules, and centrioles. Even below this level are other parts so small that they are formally classified as macromolecules (large molecules).
A critically important macromolecule—arguably “second in importance only to DNA”—is ATP. ATP is a complex nanomachine that serves as the primary energy currency of the cell (Trefil, 1992, p.93). A nanomachine is a complex precision microscopic-sized machine that fits the standard definition of a machine. ATP is the “most widely distributed high-energy compound within the human body” (Ritter, 1996, p. 301). This ubiquitous molecule is “used to build complex molecules, contract muscles, generate electricity in nerves, and light fireflies. All fuel sources of Nature, all foodstuffs of living things, produce ATP, which in turn powers virtually every activity of the cell and organism. Imagine the metabolic confusion if this were not so: Each of the diverse foodstuffs would generate different energy currencies and each of the great variety of cellular functions would have to trade in its unique currency” (Kornberg, 1989, p. 62).
ATP is an abbreviation for adenosine triphosphate, a complex molecule that contains the nucleoside adenosine and a tail consisting of three phosphates. (See Figure 1 for a simple structural formula and a space filled model of ATP.) As far as known, all organisms from the simplest bacteria to humans use ATP as their primary energy currency. The energy level it carries is just the right amount for most biological reactions. Nutrients contain energy in low-energy covalent bonds which are not very useful to do most of kinds of work in the cells.
Figure 1. Views of ATP and related structures.
These low energy bonds must be translated to high energy bonds, and this is a role of ATP. A steady supply of ATP is so critical that a poison which attacks any of the proteins used in ATP production kills the organism in minutes. Certain cyanide compounds, for example, are poisonous because they bind to the copper atom in cytochrome oxidase. This binding blocks the electron transport system in the mitochondria where ATP manufacture occurs (Goodsell, 1996, p.74).
How ATP Transfers Energy
Energy is usually liberated from the ATP molecule to do work in the cell by a reaction that removes one of the phosphate-oxygen groups, leaving adenosine diphosphate (ADP). When the ATP converts to ADP, the ATP is said to be spent. Then the ADP is usually immediately recycled in the mitochondria where it is recharged and comes out again as ATP. In the words of Trefil (1992, p. 93) “hooking and unhooking that last phosphate [on ATP] is what keeps the whole world operating.”
The enormous amount of activity that occurs inside each of the approximately one hundred trillion human cells is shown by the fact that at any instant each cell contains about one billion ATP molecules. This amount is sufficient for that cell’s needs for only a few minutes and must be rapidly recycled. Given a hundred trillion cells in the average male, about 1023 or one sextillion ATP molecules normally exist in the body. For each ATP “the terminal phosphate is added and removed 3 times each minute” (Kornberg, 1989, p. 65).
The total human body content of ATP is only about 50 grams, which must be constantly recycled every day. The ultimate source of energy for constructing ATP is food; ATP is simply the carrier and regulation-storage unit of energy. The average daily intake of 2,500 food calories translates into a turnover of a whopping 180 kg (400 lbs) of ATP (Kornberg, 1989, p. 65).
The Structure of ATP
ATP contains the purine base adenine and the sugar ribose which together form the nucleoside adenosine. The basic building blocks used to construct ATP are carbon, hydrogen, nitrogen, oxygen, and phosphorus which are assembled in a complex that contains the number of subatomic parts equivalent to over 500 hydrogen atoms. One phosphate ester bond and two phosphate anhydride bonds hold the three phosphates (PO4) and the ribose together. The construction also contains a b-N glycoside bond holding the ribose and the adenine together.
Phosphates are well-known high-energy molecules, meaning that comparatively high levels of energy are released when the phosphate groups are removed. Actually, the high energy content is not the result of simply the phosphate bond but the total interaction of all the atoms within the ATP molecule.
Because the amount of energy released when the phosphate bond is broken is very close to that needed by the typical biological reaction, little energy is wasted. Generally, ATP is connected to another reaction—a process called coupling which means the two reactions occur at the same time and at the same place, usually utilizing the same enzyme complex. Release of phosphate from ATP is exothermic (a reaction that gives off heat) and the reaction it is connected to is endothermic (requires energy input in order to occur). The terminal phosphate group is then transferred by hydrolysis to another compound, a process called phosphorylation, producing ADP, phosphate (Pi) and energy.
Figure 2. The two-dimensional stick model of adenosine phosphate family of molecules, showing the atom and bond arrangement.
The self-regulation system of ATP has been described as follows:
The high-energy bonds of ATP are actually rather unstable bonds. Because they are unstable, the energy of ATP is readily released when ATP is hydrolyzed in cellular reactions. Note that ATP is an energy-coupling agent and not a fuel. It is not a storehouse of energy set aside for some future need. Rather it is produced by one set of reactions and is almost immediately consumed by another. ATP is formed as it is needed, primarily by oxidative processes in the mitochondria. Oxygen is not consumed unless ADP and a phosphate molecule are available, and these do not become available until ATP is hydrolyzed by some energy-consuming process. Energy metabolism is therefore mostly self-regulating (Hickman, Roberts, and Larson, 1997, p.43). [Italics mine]
ATP is not excessively unstable, but it is designed so that its hydrolysis is slow in the absence of a catalyst. This insures that its stored energy is “released only in the presence of the appropriate enzyme” (McMurry and Castellion, 1996, p. 601).
The Function of ATP
The ATP is used for many cell functions including transport work moving substances across cell membranes. It is also used for mechanical work, supplying the energy needed for muscle contraction. It supplies energy not only to heart muscle (for blood circulation) and skeletal muscle (such as for gross body movement), but also to the chromosomes and flagella to enable them to carry out their many functions. A major role of ATP is in chemical work, supplying the needed energy to synthesize the multi-thousands of types of macromolecules that the cell needs to exist.
ATP is also used as an on-off switch both to control chemical reactions and to send messages. The shape of the protein chains that produce the building blocks and other structures used in life is mostly determined by weak chemical bonds that are easily broken and remade. These chains can shorten, lengthen, and change shape in response to the input or withdrawal of energy. The changes in the chains alter the shape of the protein and can also alter its function or cause it to become either active or inactive.
The ATP molecule can bond to one part of a protein molecule, causing another part of the same molecule to slide or move slightly which causes it to change its conformation, inactivating the molecule. Subsequent removal of ATP causes the protein to return to its original shape, and thus it is again functional. The cycle can be repeated until the molecule is recycled, effectively serving as an on and off switch (Hoagland and Dodson, 1995, p.104). Both adding a phosphorus (phosphorylation) and removing a phosphorus from a protein (dephosphorylation) can serve as either an on or an off switch.
How is ATP Produced?
ATP is manufactured as a result of several cell processes including fermentation, respiration and photosynthesis. Most commonly the cells use ADP as a precursor molecule and then add a phosphorus to it. In eukaryotes this can occur either in the soluble portion of the cytoplasm (cytosol) or in special energy-producing structures called mitochondria. Charging ADP to form ATP in the mitochondria is called chemiosmotic phosphorylation. This process occurs in specially constructed chambers located in the mitochondrion’s inner membranes.
Figure 3. An Outline of the ATP-synthase macro-molecule showing its subunits and nanomachine traits. ATP-synthase converts ADP into ATP, a process called charging. Shown behind ATP-synthase is the membrane in which the ATP-synthase is mounted. For the ATP that is charged in the mitochondria, ATP-synthase is located in the inner membrane.
The mitochondrion itself functions to produce an electrical chemical gradient—somewhat like a battery—by accumulating hydrogen ions in the space between the inner and outer membrane. This energy comes from the estimated 10,000 enzyme chains in the membranous sacks on the mitochondrial walls. Most of the food energy for most organisms is produced by the electron transport chain. Cellular oxidation in the Krebs cycle causes an electron build-up that is used to push H+ ions outward across the inner mitochondrial membrane (Hickman et al., 1997, p. 71).
As the charge builds up, it provides an electrical potential that releases its energy by causing a flow of hydrogen ions across the inner membrane into the inner chamber. The energy causes an enzyme to be attached to ADP which catalyzes the addition of a third phosphorus to form ATP. Plants can also produce ATP in this manner in their mitochondria but plants can also produce ATP by using the energy of sunlight in chloroplasts as discussed later. In the case of eukaryotic animals the energy comes from food which is converted to pyruvate and then to acetyl coenzyme A (acetyl CoA). Acetyl CoA then enters the Krebs cycle which releases energy that results in the conversion of ADP back into ATP.
How does this potential difference serve to reattach the phosphates on ADP molecules? The more protons there are in an area, the more they repel each other. When the repulsion reaches a certain level, the hydrogens ions are forced out of a revolving-door-like structure mounted on the inner mitochondria membrane called ATP synthase complexes. This enzyme functions to reattach the phosphates to the ADP molecules, again forming ATP.
The ATP synthase revolving door resembles a molecular water wheel that harnesses the flow of hydrogen ions in order to build ATP molecules. Each revolution of the wheel requires the energy of about nine hydrogen ions returning into the mitochondrial inner chamber (Goodsell, 1996, p.74). Located on the ATP synthase are three active sites, each of which converts ADP to ATP with every turn of the wheel. Under maximum conditions, the ATP synthase wheel turns at a rate of up to 200 revolutions per second, producing 600 ATPs during that second.
ATP is used in conjunction with enzymes to cause certain molecules to bond together. The correct molecule first docks in the active site of the enzyme along with an ATP molecule. The enzyme then catalyzes the transfer of one of the ATP phosphates to the molecule, thereby transferring the energy stored in the ATP molecule. Next a second molecule docks nearby at a second active site on the enzyme. The phosphate is then transferred to it, providing the energy needed to bond the two molecules now attached to the enzyme. Once they are bonded, the new molecule is released. This operation is similar to using a mechanical jig to properly position two pieces of metal which are then welded together. Once welded, they are released as a unit and the process then can begin again.
A Double Energy Packet
Although ATP contains the amount of energy necessary for most reactions, at times more energy is required. The solution is for ATP to release two phosphates instead of one, producing an adenosine monophosphate (AMP) plus a chain of two phosphates called a pyrophosphate. How adenosine monophosphate is built up into ATP again illustrates the precision and the complexity of the cell energy system. The enzymes used in glycolysis, the citric acid cycle, and the electron transport system, are all so precise that they will replace only a single phosphate. They cannot add two new phosphates to an AMP molecule to form ATP.
The solution is an intricate enzyme called adenylate kinase which transfers a single phosphate from an ATP to the AMP, producing two ADP molecules. The two ADP molecules can then enter the normal Krebs cycle designed to convert ADP into ATP. Adenylate kinase requires an atom of magnesium—and this is one of the reasons why sufficient dietary magnesium is important.
Adenylate kinase is a highly organized but compact enzyme with its active site located deep within the molecule. The deep active site is required because the reactions it catalyzes are sensitive to water. If water molecules lodged between the ATP and the AMP, then the phosphate might break ATP into ADP and a free phosphate instead of transferring a phosphate from ATP to AMP to form ADP.
To prevent this, adenylate kinase is designed so that the active site is at the end of a channel deep in the structure which closes around AMP and ATP, shielding the reaction from water. Many other enzymes that use ATP rely on this system to shelter their active site to prevent inappropriate reactions from occurring. This system ensures that the only waste that occurs is the normal wear, tear, repair, and replacement of the cell’s organelles.
Pyrophosphates and pyrophosphoric acid, both inorganic forms of phosphorus, must also be broken down so they can be recycled. This phosphate breakdown is accomplished by the inorganic enzyme pyrophosphatase which splits the pyrophosphate to form two free phosphates that can be used to charge ATP (Goodsell, 1996, p.79). This system is so amazingly efficient that it produces virtually no waste, which is astounding considering its enormously detailed structure. Goodsell (1996, p. 79) adds that “our energy-producing machinery is designed for the production of ATP; quickly, efficiently, and in large quantity.”
The main energy carrier the body uses is ATP, but other energized nucleotides are also utilized such as thymine, guanine, uracil, and cytosine for making RNA and DNA. The Krebs cycle charges only ADP, but the energy contained in ATP can be transferred to one of the other nucleosides by means of an enzyme called nucleoside diphosphate kinase. This enzyme transfers the phosphate from a nucleoside triphosphate, commonly ATP, to a nucleoside diphosphate such as guanosine diphosphate (GDP) to form guanosine triphosphate (GTP).
The nucleoside diphosphate kinase works by one of its six active sites binding nucleoside triphosphate and releasing the phosphate which is bonded to a histidine. Then the nucleoside triphosphate, which is now a diphosphate, is released, and a different nucleoside diphosphate binds to the same site—and as a result the phosphate that is bonded to the enzyme is transferred, forming a new triphosphate. Scores of other enzymes exist in order for ATP to transfer its energy to the various places where it is needed. Each enzyme must be specifically designed to carry out its unique function, and most of these enzymes are critical for health and life.
The body does contain some flexibility, and sometimes life is possible when one of these enzymes is defective—but the person is often handicapped. Also, back-up mechanisms sometimes exist so that the body can achieve the same goals through an alternative biochemical route. These few simple examples eloquently illustrate the concept of over-design built into the body. They also prove the enormous complexity of the body and its biochemistry.
The Message of the MoleculeWithout ATP, life as we understand it could not exist. It is a perfectly-designed, intricate molecule that serves a critical role in providing the proper size energy packet for scores of thousands of classes of reactions that occur in all forms of life. Even viruses rely on an ATP molecule identical to that used in humans. The ATP energy system is quick, highly efficient, produces a rapid turnover of ATP, and can rapidly respond to energy demand changes (Goodsell, 1996, p.79).
Furthermore, the ATP molecule is so enormously intricate that we are just now beginning to understand how it works. Each ATP molecule is over 500 atomic mass units (500 u). In manufacturing terms, the ATP molecule is a machine with a level of organization on the order of a research microscope or a standard television (Darnell, Lodish, and Baltimore, 1996).
Among the questions evolutionists must answer include the following, “How did life exist before ATP?” “How could life survive without ATP since no form of life we know of today can do that?” and “How could ATP evolve and where are the many transitional forms required to evolve the complex ATP molecule?” No feasible candidates exist and none can exist because only a perfect ATP molecule can properly carry out its role in the cell.
In addition, a potential ATP candidate molecule would not be selected for by evolution until it was functional and life could not exist without ATP or a similar molecule that would have the same function. ATP is an example of a molecule that displays irreducible complexity which cannot be simplified and still function (Behe, 1996). ATP could have been created only as a unit to function immediately in life and the same is true of the other intricate energy molecules used in life such as GTP.
Although other energy molecules can be used for certain cell functions, none can even come close to satisfactorily replacing all the many functions of ATP. Over 100,000 other detailed molecules like ATP have also been designed to enable humans to live, and all the same problems related to their origin exist for them all. Many macromolecules that have greater detail than ATP exist, as do a few that are less highly organized, and in order for life to exist all of them must work together as a unit.
The Contrast between Prokaryotic and
Eukaryotic ATP Production
An enormous gap exists between prokaryote (bacteria and cyanobacteria) cells and eukaryote (protists, plants and animals) type of cells:
...prokaryotes and eukaryotes are profoundly different from each other and clearly represent a marked dichotomy in the evolution of life. . . The organizational complexity of the eukaryotes is so much greater than that of the prokaryotes that it is difficult to visualize how a eukaryote could have arisen from any known prokaryote (Hickman et al., 1997, p. 39).
Some of the differences are that prokaryotes lack organelles, a cytoskeleton, and most of the other structures present in eukaryotic cells. Consequently, the functions of most organelles and other ultrastructure cell parts must be performed in bacteria by the cell membrane and its infoldings called mesosomes.
The Four Major Methods of Producing ATP
A crucial difference between prokaryotes and eukaryotes is the means they use to produce ATP. All life produces ATP by three basic chemical methods only: oxidative phosphorylation, photophosphorylation, and substrate-level phosphorylation (Lim, 1998, p. 149). In prokaryotes ATP is produced both in the cell wall and in the cytosol by glycolysis. In eukaryotes most ATP is produced in chloroplasts (for plants), or in mitochondria (for both plants and animals). No means of producing ATP exists that is intermediate between these four basic methods and no transitional forms have ever been found that bridge the gap between these four different forms of ATP production. The machinery required to manufacture ATP is so intricate that viruses are not able to make their own ATP. They require cells to manufacture it and viruses have no source of energy apart from cells.
In prokaryotes the cell membrane takes care of not only the cell’s energy-conversion needs, but also nutrient processing, synthesizing of structural macromolecules, and secretion of the many enzymes needed for life (Talaro and Talaro, 1993, p. 77). The cell membrane must, for this reason be compared with the entire eukaryote cell ultrastructure which performs these many functions. No simple means of producing ATP is known and prokaryotes are not by any means simple. They contain over 5,000 different kinds of molecules and can use sunlight, organic compounds such as carbohydrates and inorganic compounds as sources of energy to manufacture ATP.
Another example of the cell membrane in prokaryotes assuming a function of the eukaryotic cell ultrastructure is as follows: Their DNA is physically attached to the bacterial cell membrane and DNA replication may be initiated by changes in the membrane. These membrane changes are in turn related to the bacterium’s growth. Further, the mesosome appears to guide the duplicated chromatin bodies into the two daughter cells during cell division (Talaro and Talaro, 1993).
In eukaryotes the mitochondria produce most of the cell’s ATP (anaerobic glycolysis also produces some) and in plants the chloroplasts can also service this function. The mitochondria produce ATP in their internal membrane system called the cristae. Since bacteria lack mitochondria, as well as an internal membrane system, they must produce ATP in their cell membrane which they do by two basic steps. The bacterial cell membrane contains a unique structure designed to produce ATP and no comparable structure has been found in any eukaryotic cell (Jensen, Wright, and Robinson, 1997).
In bacteria, the ATPase and the electron transport chain are located inside the cytoplasmic membrane between the hydrophobic tails of the phospholipid membrane inner and outer walls. Breakdown of sugar and other food causes the positively charged protons on the outside of the membrane to accumulate to a much higher concentration than they are on the membrane inside. This creates an excess positive charge on the outside of the membrane and a relatively negative charge on the inside.
The result of this charge difference is a dissociation of H2O molecules into H+ and OH– ions. The H+ ions that are produced are then transported outside of the cell and the OH– ions remain on the inside. This results in a potential energy gradient similar to that produced by charging a flashlight battery. The force the potential energy gradient produces is called a proton motive force that can accomplish a variety of cell tasks including converting ADP into ATP.
In some bacteria such as Halobacterium this system is modified by use of bacteriorhodopsin, a protein similar to the sensory pigment rhodopsin used in the vertebrate retina (Lim, 1998, p. 166). Illumination causes the pigment to absorb light energy, temporarily changing rhodopsin from a trans to a cis form. The trans to cis conversion causes deprotonation and the transfer of protons across the plasma membrane to the periplasm.
The proton gradient that results is used to drive ATP synthesis by use of the ATPase complex. This modification allows bacteria to live in low oxygen but rich light regions. This anaerobic ATP manufacturing system, which is unique to prokaryotes, uses a chemical compound other than oxygen as a terminal electron acceptor (Lim, 1998, p. 168). The location of the ATP producing system is only one of many major contrasts that exist between bacterial cell membranes and mitochondria.
ChloroplastsChloroplasts are double membraned ATP-producing organelles found only in plants. Inside their outer membrane is a set of thin membranes organized into flattened sacs stacked up like coins called thylakoids (Greek thylac or sack, and oid meaning like). The disks contain chlorophyll pigments that absorb solar energy which is the ultimate source of energy for all the plant’s needs including manufacturing carbohydrates from carbon dioxide and water (Mader, 1996, p. 75). The chloroplasts first convert the solar energy into ATP stored energy, which is then used to manufacture storage carbohydrates which can be converted back into ATP when energy is needed.
The chloroplasts also possess an electron transport system for producing ATP. The electrons that enter the system are taken from water. During photosynthesis, carbon dioxide is reduced to a carbohydrate by energy obtained from ATP (Mader, 1996, p. 12). Photosynthesizing bacteria (cyanobacteria) use yet another system. Cyanobacteria do not manufacture chloroplasts but use chlorophyll bound to cytoplasmic thylakoids. Once again plausible transitional forms have never been found that can link these two forms of ATP production from the photosynthesis system.
The two most common evolutionary theories of the origin of the mitochondria-chloroplast ATP production system are 1) endosymbiosis of mitochondria and chloroplasts from the bacterial membrane system and 2) the gradual evolution of the prokaryote cell membrane system of ATP production into the mitochondria and chloroplast systems. Believers in endosymbiosis teach that mitochondria were once free-living bacteria, and that “early in evolution ancestral eukaryotic cells simply ate their future partners” (Vogel, 1998, p. 1633). Both the gradual conversion and endosymbiosis theory require many transitional forms, each new one which must provide the animal with a competitive advantage compared with the unaltered animals.
The many contrasts between the prokaryotic and eukaryotic means of producing ATP, some of which were noted above, are strong evidence against the endosymbiosis theory. No intermediates to bridge these two systems has ever been found and arguments put forth in the theory’s support are all highly speculative. These and other problems have recently become more evident as a result of recent major challenges to the standard endosymbiosis theory. The standard theory has recently been under attack from several fronts, and some researchers are now arguing for a new theory:
Scientists pondering how the first complex cell came together say the new idea could solve some nagging problems with the prevailing theory... “[the new theory is]... elegantly argued,” says Michael Gray of Dalhouisie University in Halifax, Nova Scotia, but “there are an awful lot of things the hypothesis doesn’t account for.” In the standard picture of eukaryote evolution, the mitochondrion was a lucky accident. First, the ancestral cell—probably an archaebacterium, recent genetic analyses suggest—acquired the ability to engulf and digest complex molecules. It began preying on its microbial companions. At some point, however, this predatory cell didn’t fully digest its prey, and an even more successful cell resulted when an intended meal took up permanent residence and became the mitochondrion. For years, scientists had thought they had examples of the direct descendants of those primitive eukaryotes: certain protists that lack mitochondria. But recent analysis of the genes in those organisms suggests that they, too, once carried mitochondria but lost them later (Science, 12 September 1997, p. 1604). These findings hint that eukaryotes might somehow have acquired their mitochondria before they had evolved the ability to engulf and digest other cells (Vogel, 1998, p. 1633).
Summary
In this brief review we have examined only one cell macromolecule, ATP, and the intricate mechanisms which produce it. We have also looked at the detailed supporting mechanism which allows the ATP molecule to function. ATP is only one of hundreds of thousands of essential molecules, each one that has a story. As each of those stories is told, they will stand as a tribute to both the genius and the enormously complex design of the natural world. All the books in the largest library in the world may not be able to contain the information needed to understand and construct the estimated 100,000 complex macromolecule machines used in humans. Much progress has been made in understanding the structure and function of organic macromolecules and some of the simpler ones are now being manufactured by pharmaceutical firms.
Now that scientists understand how some of these highly organized molecules function and why they are required for life, their origin must be explained. We know only four basic methods of producing ATP: in bacterial cell walls, in the cytoplasm by photosynthesis, in chloroplasts, and in mitochondria. No transitional forms exist to bridge these four methods by evolution. According to the concept of irreducible complexity, these ATP producing machines must have been manufactured as functioning units and they could not have evolved by Darwinism mechanisms. Anything less than an entire ATP molecule will not function and a manufacturing plant which is less then complete cannot produce a functioning ATP. Some believe that the field of biochemistry which has achieved this understanding has already falsified the Darwinian world view (Behe, 1996).*
References
Behe, Michael. 1996. Darwin’s black box: The biochemical challenge to evolution. The Free Press. New York.
Darnell, James, Harvey Lodish, and David Baltimore. 1996. Molecular cell biology, 3rd edition. W.H. Freeman. New York.
Goodsell, David S. 1996. Our molecular nature. Springer-Verlag. New York.
Hickman, Cleveland P. 1997. Integrated principles of zoology, 10th edition. William C. Brown/McGraw Hill. New York.
Hickman, Cleveland P., Larry Roberts, and Allan Larson. 1997. The biology of animals, 7th edition. William C. Brown/McGraw Hill. New York.
Hoagland, Mahlon and Bert Dodson. 1995. The way life works. Random House. New York.
Jensen, Marcus, Donald Wright, and Richard Robinson. 1997. Microbiology for the health sciences, 4th edition. Prentice-Hall. Upper Saddle River, NJ.
Kornberg, Arthur. 1989. For the love of enzymes. Harvard University Press. Cambridge, MA.
Lim, Daniel. 1998. Microbiology, 2nd edition. William C. Brown/McGraw Hill. New York.
Mader, Sylvia. 1996. Biology, 6th edition. William C. Brown. Dubuque, IA.
McMurry, John and Mary Castellion. 1996. Fundamentals of general, organic, and biological chemistry, 2nd edition. Prentice Hall. Upper Saddle River, NJ.
Ritter, Peck. 1996. Biochemistry, a foundation. Brooks/Cole. Pacific Grove CA.
Talaro, Kathleen and Arthur Talaro. 1993. Foundations in microbiology. William C. Brown. Dubuque, IA.
Trefil, James. 1992. 1001 Things everyone should know about science. Doubleday. New York.
Vogel, Gretchen. 1998. Did the first complex cell eat hydrogen? Science 279: 1633-1634.